Quinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
- The U.S. Food and Drug Administration said a voluntary recall has been issued for blood pressure medications that have shown the presence of impurities that could increase the risk of cancer.for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Nitrosamine impurities are regularly found in foods like cured or grilled meats, vegetables and dairy products, according to the FDA.Lupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Blood pressure tablets recalled over potential cancer risk, FDA announcesFour lots of the blood pressure medication Quinapril were voluntarily recalled over concerns it could increase the risk of cancer, the Food and Drug Administration said.
Blood pressure tablets recalled over potential cancer risk, FDA announcesFour lots of the blood pressure medication Quinapril were voluntarily recalled over concerns it could increase the risk of cancer, the Food and Drug Administration said.
Weiterlesen »
 Blood pressure tablets recalled over potential cancer risk, FDA announcesThe FDA announced the second recall in months of blood-pressure medication over an elevated cancer risk. This time Quinapril tablets are being pulled.
Blood pressure tablets recalled over potential cancer risk, FDA announcesThe FDA announced the second recall in months of blood-pressure medication over an elevated cancer risk. This time Quinapril tablets are being pulled.
Weiterlesen »
 Blood pressure tablets recalled over potential cancer risk, FDA announcesPatients who are on the medication don’t need to stop taking it immediately, but are advised to discuss an alternative treatment with their healthcare provider.
Blood pressure tablets recalled over potential cancer risk, FDA announcesPatients who are on the medication don’t need to stop taking it immediately, but are advised to discuss an alternative treatment with their healthcare provider.
Weiterlesen »
 FDA: Blood pressure medicine recalled over cancer riskQuinapril is used to treat hypertension and lower blood pressure.
FDA: Blood pressure medicine recalled over cancer riskQuinapril is used to treat hypertension and lower blood pressure.
Weiterlesen »
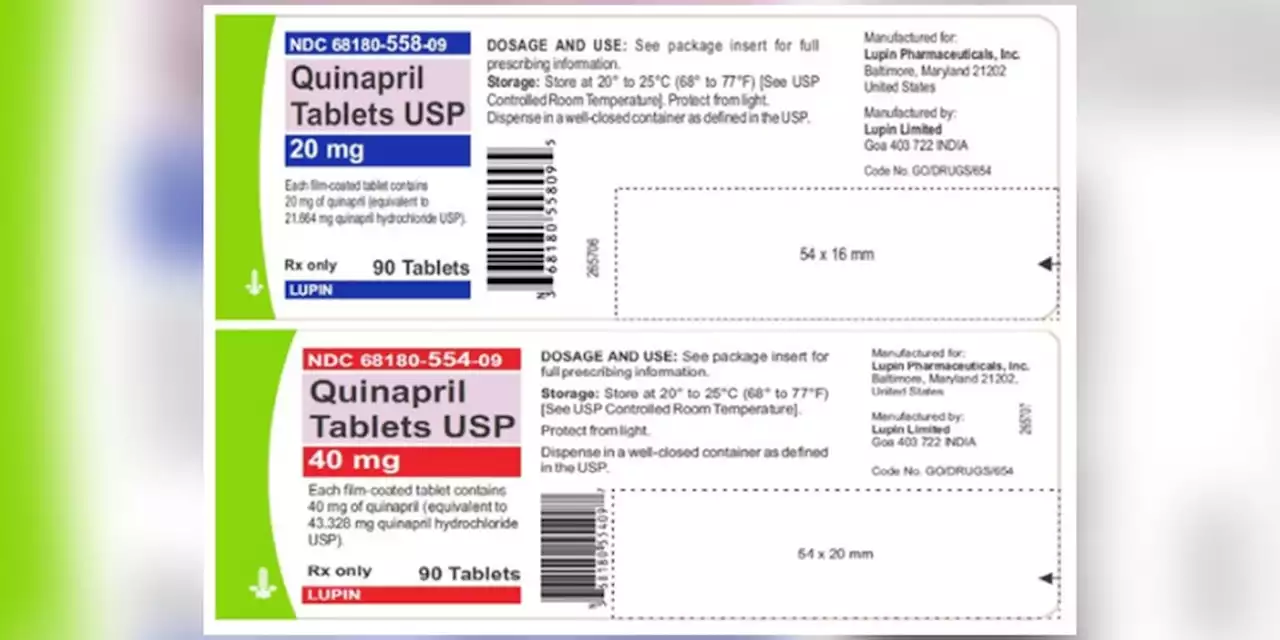 Blood pressure tablets recalled due to potential cancer risks, FDA saysQuinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
Blood pressure tablets recalled due to potential cancer risks, FDA saysQuinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
Weiterlesen »
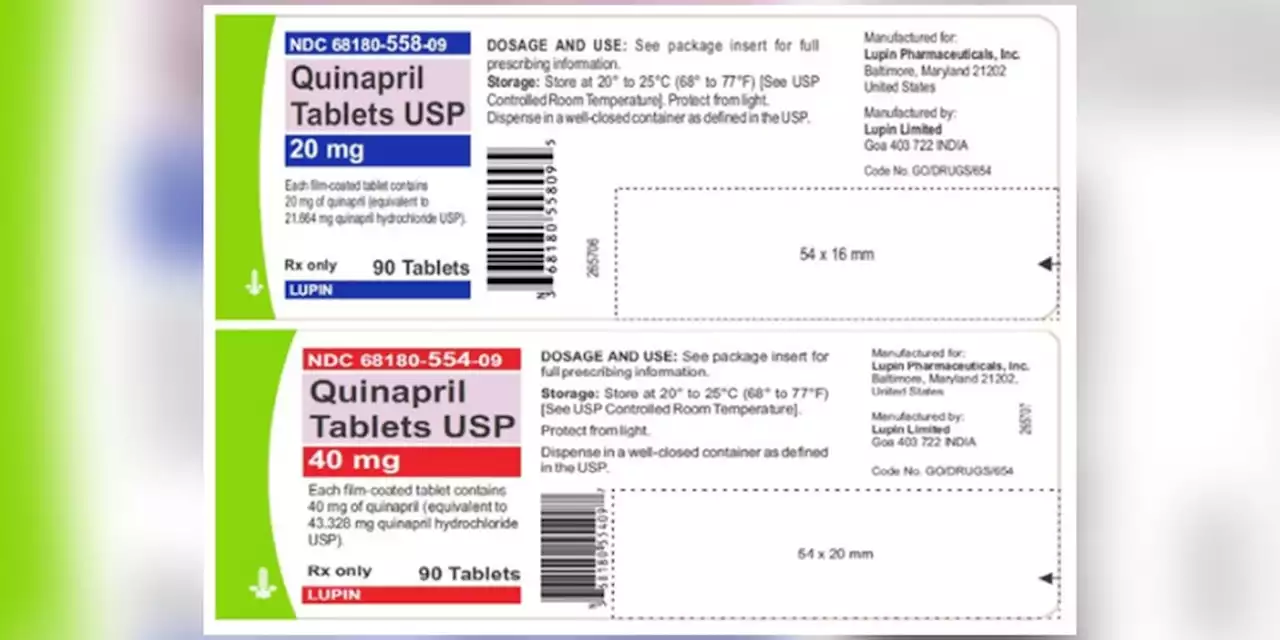 Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Weiterlesen »
