The US Food and Drug Administration is postponing an advisory panel meeting on authorizing Pfizer’s Covid-19 vaccine in children under 5.
Pfizer and BioNTech filed a request with the US Food and Drug Administration in the first week of February for an emergency use authorization of their vaccine in children 6 months to 5 years old. The FDA's Vaccines and Related Biological Products Advisory Committee scheduled a meeting for February 15 to go over data from trials of the vaccines in young children and make a recommendation on their use.
"We take our responsibility for reviewing these vaccines very seriously because we're parents as well, and in looking over the data, I think parents can feel reassured that we have set a standard by which we feel that if something does not meet that standard, we can't proceed forward," Marks said.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
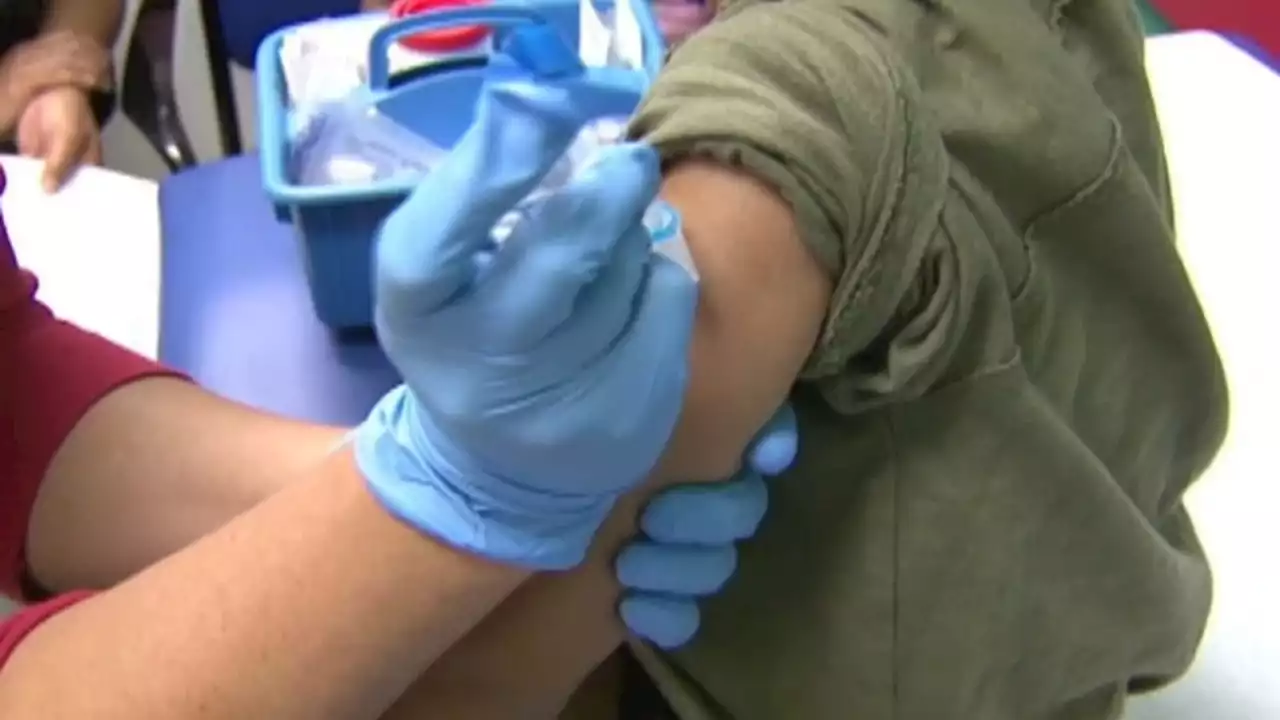 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataBREAKING: The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataBREAKING: The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Weiterlesen »
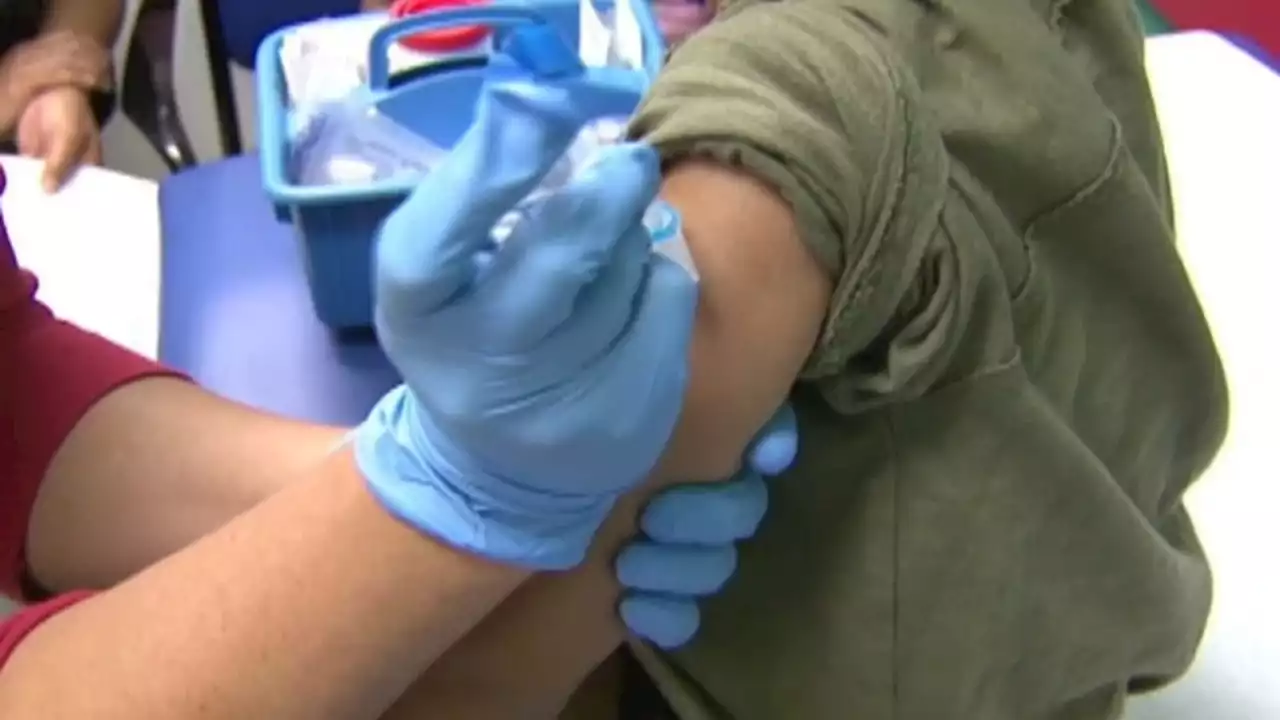 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Weiterlesen »
 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Weiterlesen »
 FDA postpones advisory panel meeting on Pfizer Covid-19 vaccine for younger childrenThe US Food and Drug Administration is postponing an advisory panel meeting on authorizing Pfizer’s Covid-19 vaccine in children under 5.
FDA postpones advisory panel meeting on Pfizer Covid-19 vaccine for younger childrenThe US Food and Drug Administration is postponing an advisory panel meeting on authorizing Pfizer’s Covid-19 vaccine in children under 5.
Weiterlesen »
