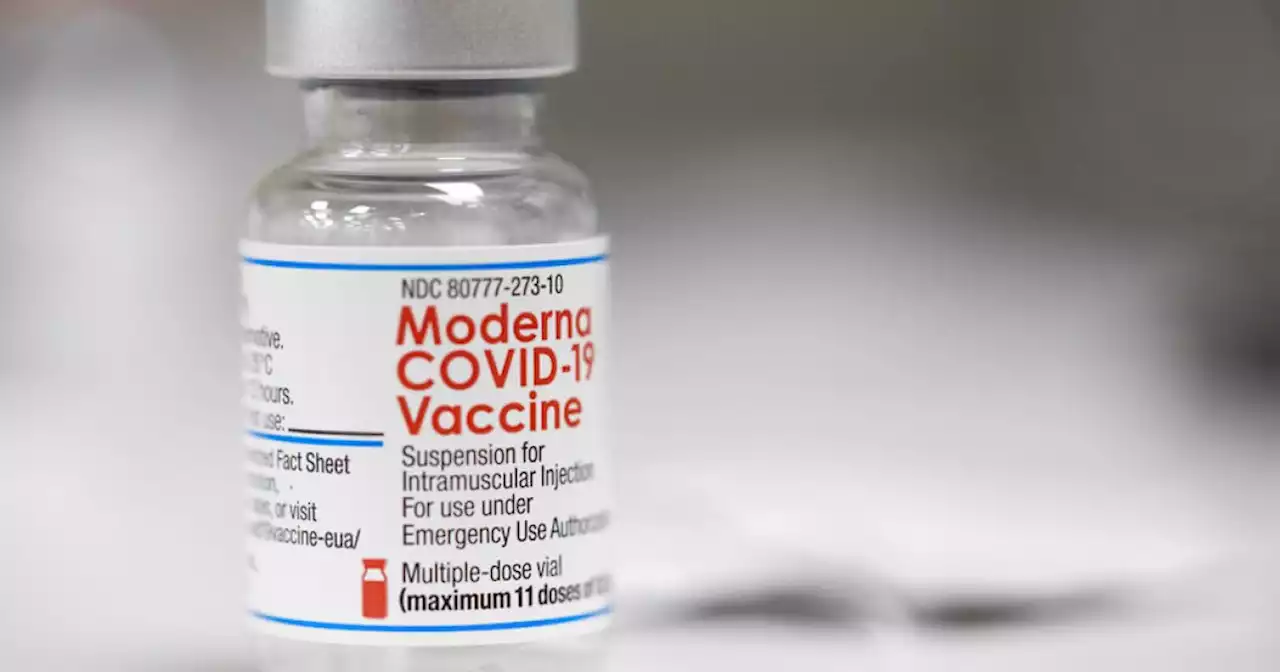
A government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
The expert panel agreed Tuesday that the vaccine made by Moderna is safe and effective enough to give to U.S. kids ages 6 to 17.The Food and Drug Administration will consider the panel's advice and decide whether to authorize the shots.
The same FDA panel will meet Wednesday to consider shots from Moderna and Pfizer for the littlest kids, those under 5.The FDA said they did not have any cases of the heart inflammation myocarditis pop-up in Moderna’s clinical trials in children in the listed age group, the news outlet
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Weiterlesen »
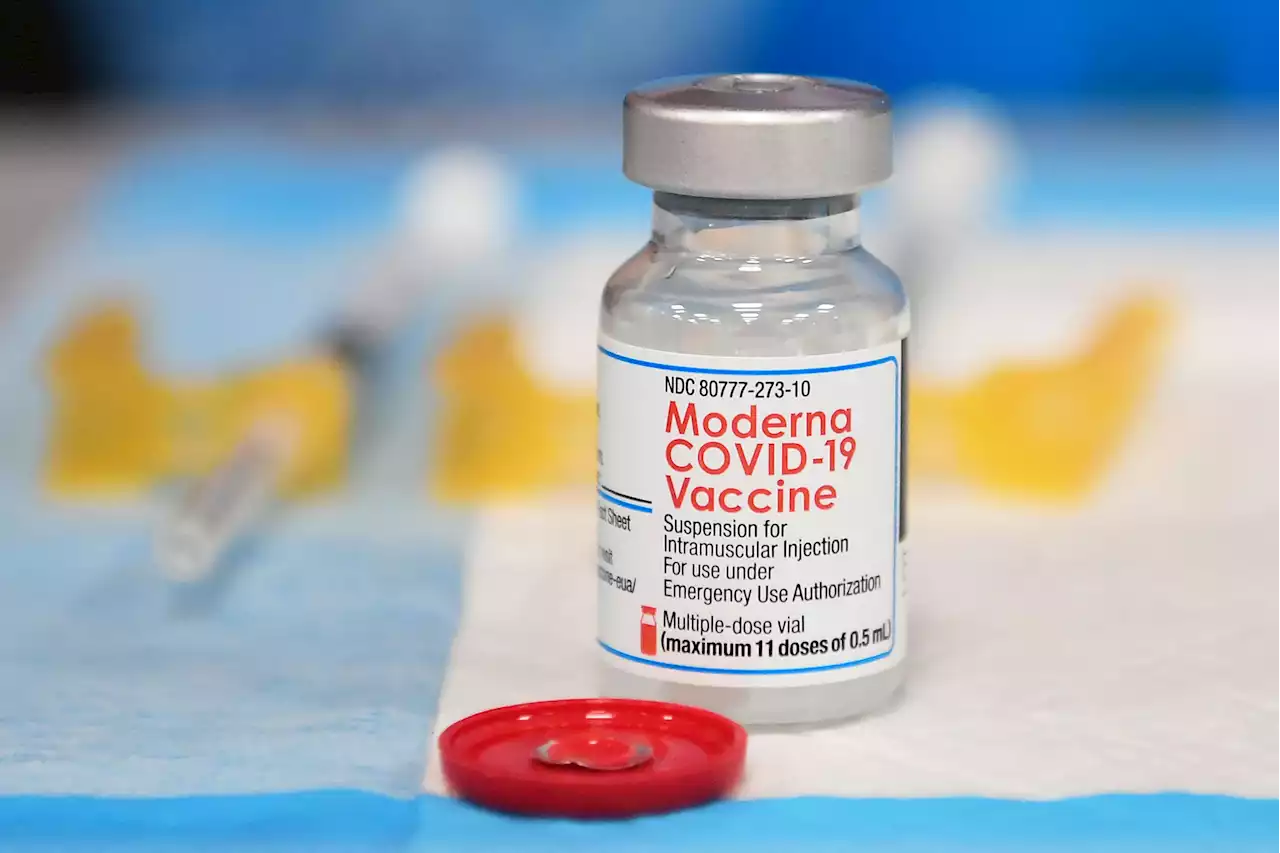 FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
Weiterlesen »
 FDA says Pfizer's COVID-19 vaccine appears effective for kids under 5Federal health officials said Sunday that kid-sizes doses of Pfizer’s COVID-19 vaccines appear to be safe and effective for kids under 5. If regulators clear the shots, vaccinations could begin as soon as next week.
FDA says Pfizer's COVID-19 vaccine appears effective for kids under 5Federal health officials said Sunday that kid-sizes doses of Pfizer’s COVID-19 vaccines appear to be safe and effective for kids under 5. If regulators clear the shots, vaccinations could begin as soon as next week.
Weiterlesen »
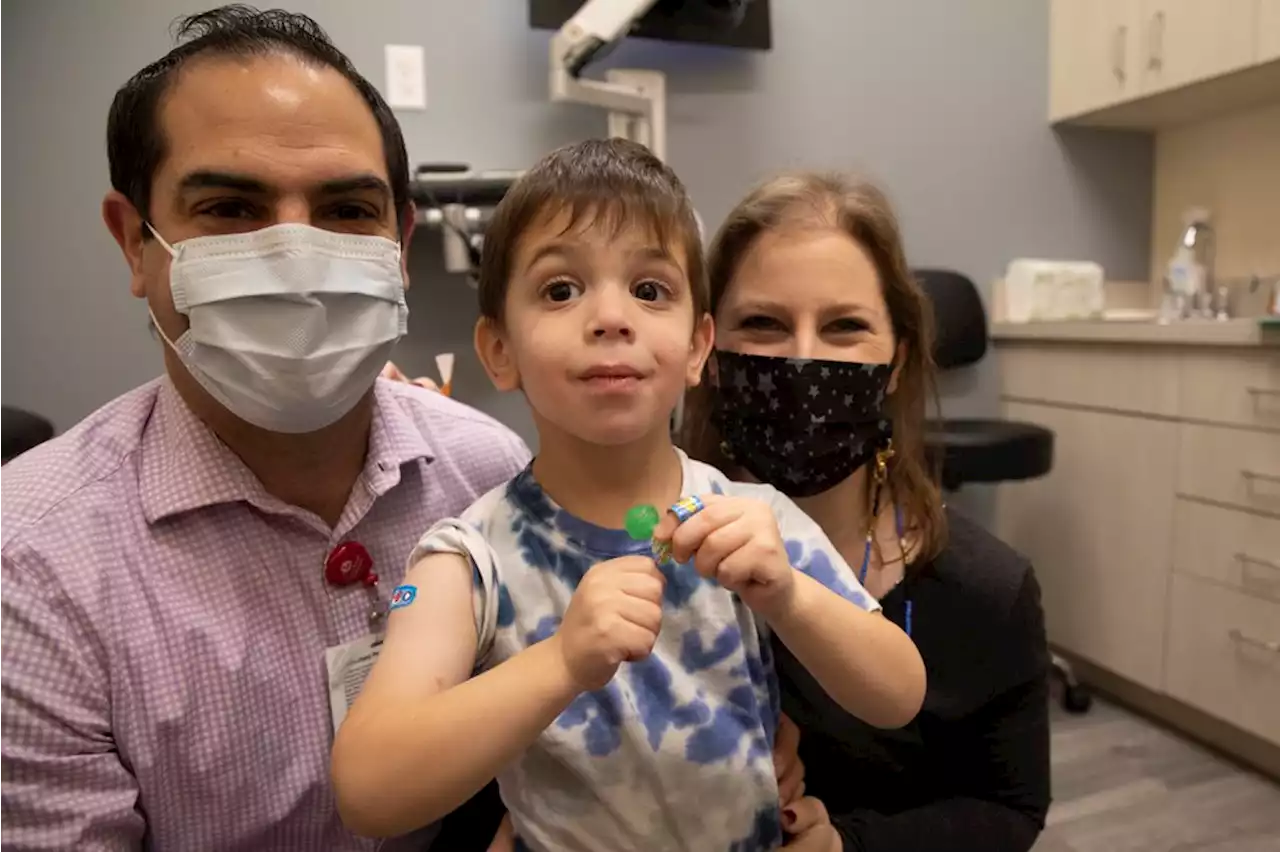 FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Weiterlesen »
 FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
Weiterlesen »
 FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
Weiterlesen »