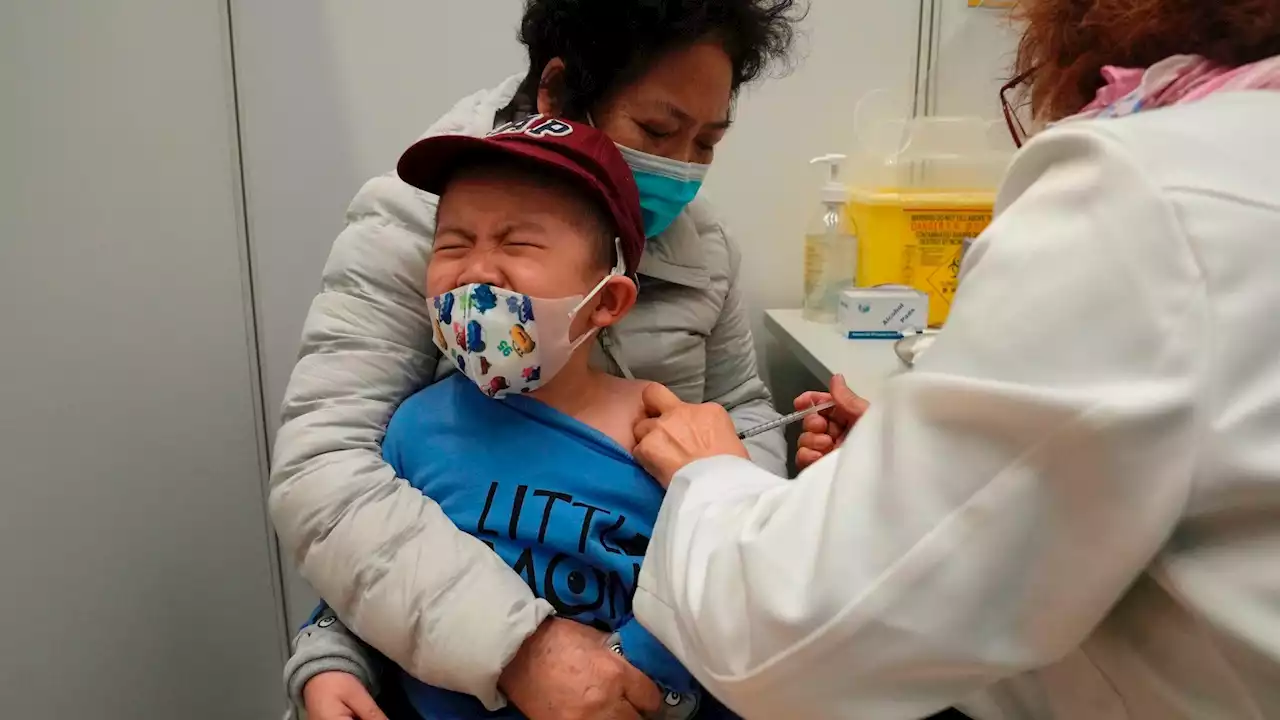
Check it out, US_FDA gave a thumbs-up for youngsters under 5 to receive Moderna and Pfizer vaccines.
The outside experts voted unanimously that the benefits of the shots outweigh any risks for children under 5 — that’s roughly 18 million youngsters. They are the last age group in the U.S. without access to COVID-19 vaccines and many parents have been anxious to protect their little children.“This is a long-awaited vaccine,” said one panel member, Dr. Jay Portnoy of Children’s Hospital in Kansas City, Missouri.
“That is a really important point,” said Dr. Jesse Goodman of Georgetown University, a former FDA vaccine chief. “You can’t compare the vaccines directly.’’ Moderna’s shots are one-quarter the dose of the company’s adult shots. Two doses appeared strong enough to prevent severe illness but only about 40% to 50% effective at preventing milder infections. Moderna has added a booster to its study and expects to eventually offer one.
The same FDA panel on Tuesday backed Moderna’s half-sized shots for ages 6 to 11 and full-sized doses for teens. If authorized by the FDA, it would be the second option for those age groups. Currently Pfizer vaccine is their only choice.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
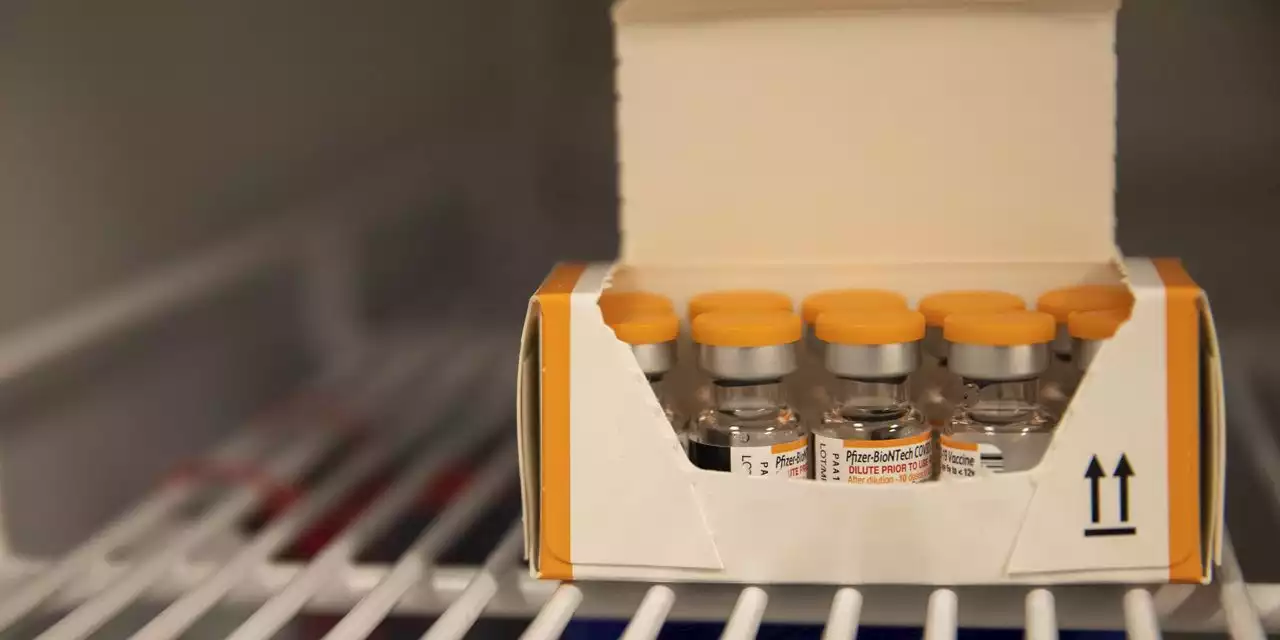 FDA Advisers Review Pfizer, Moderna Covid-19 Vaccines in Young ChildrenThe advisory panel’s meeting moves the Pfizer and Moderna shots one step closer to authorization for one of the last groups of people in the U.S. who haven’t been able to get vaccinated against the coronavirus.
FDA Advisers Review Pfizer, Moderna Covid-19 Vaccines in Young ChildrenThe advisory panel’s meeting moves the Pfizer and Moderna shots one step closer to authorization for one of the last groups of people in the U.S. who haven’t been able to get vaccinated against the coronavirus.
Weiterlesen »
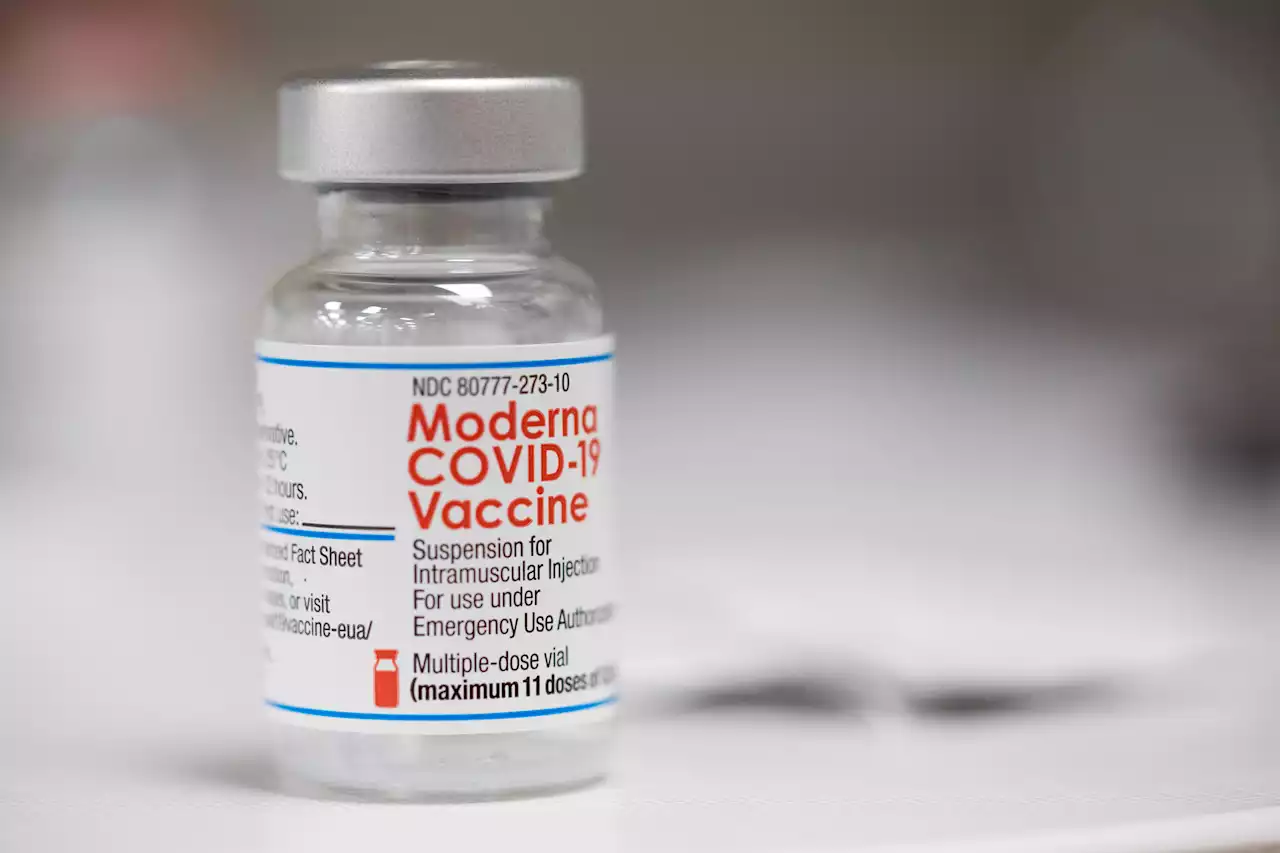
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Weiterlesen »
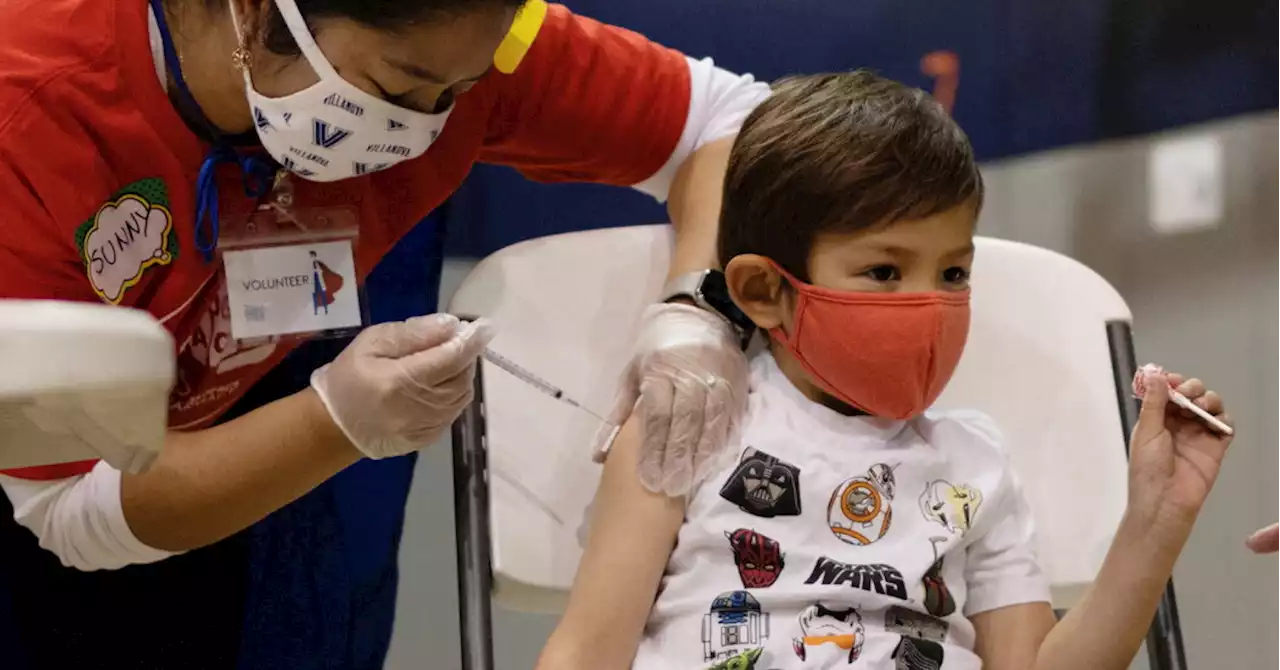
 FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
Weiterlesen »
 Pfizer Vaccine Effective in Children Under 5, the F.D.A. SaysOutside experts will make their recommendations this week on how the agency should rule on applications from Pfizer and Moderna to vaccinate the nation’s youngest children.
Pfizer Vaccine Effective in Children Under 5, the F.D.A. SaysOutside experts will make their recommendations this week on how the agency should rule on applications from Pfizer and Moderna to vaccinate the nation’s youngest children.
Weiterlesen »
 FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
Weiterlesen »
 FDA says Pfizer three-shot vaccine is safe for children under 5, ahead of panel meeting that will also review Moderna's two-shot regimenFederal health officials said Sunday that Pfizer’s COVID-19 vaccine appears safe and effective for children below the age of five. Experts will meet on Wednesday to vote on whether the shots are ready for 18 million children.
FDA says Pfizer three-shot vaccine is safe for children under 5, ahead of panel meeting that will also review Moderna's two-shot regimenFederal health officials said Sunday that Pfizer’s COVID-19 vaccine appears safe and effective for children below the age of five. Experts will meet on Wednesday to vote on whether the shots are ready for 18 million children.
Weiterlesen »