JUST IN: The FDA announced a proposed ban on menthol cigarettes and all flavors in cigars, a move that could further drive down smoking rates in the U.S.
The Food and Drug Administration announced on Thursday a proposed ban on menthol cigarettes and all flavors in cigars, a move that could further drive down smoking rates in the U.S.
. In the U.S., menthol cigarettes represent over a third of cigarette sales, with almost 19 million users. Black Americans have disproportionately high rates of menthol smoking, a consequence of years of racially targeted advertising.according to the FDA
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA Announces Recall of Popular Blood Pressure Medication Due to Potential Cancer RiskIt’s time to double-check your medicine cabinets.
FDA Announces Recall of Popular Blood Pressure Medication Due to Potential Cancer RiskIt’s time to double-check your medicine cabinets.
Weiterlesen »
 FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
Weiterlesen »
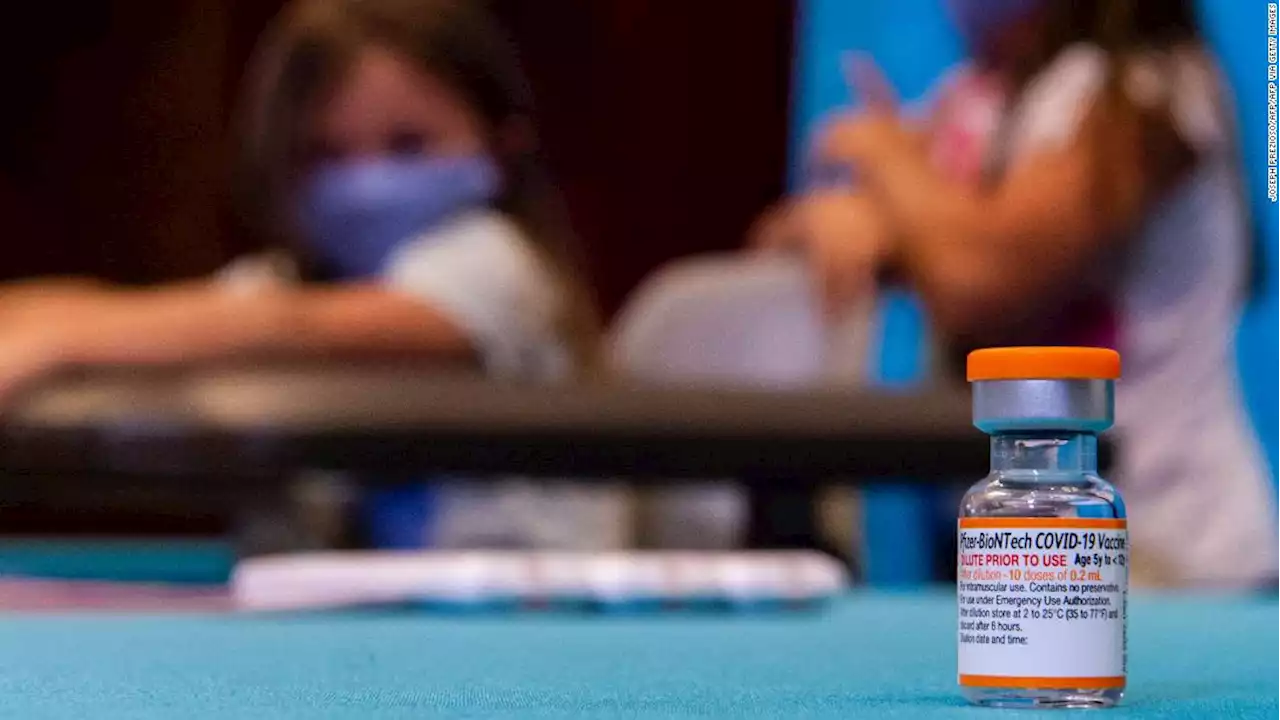 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Weiterlesen »
 Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Weiterlesen »
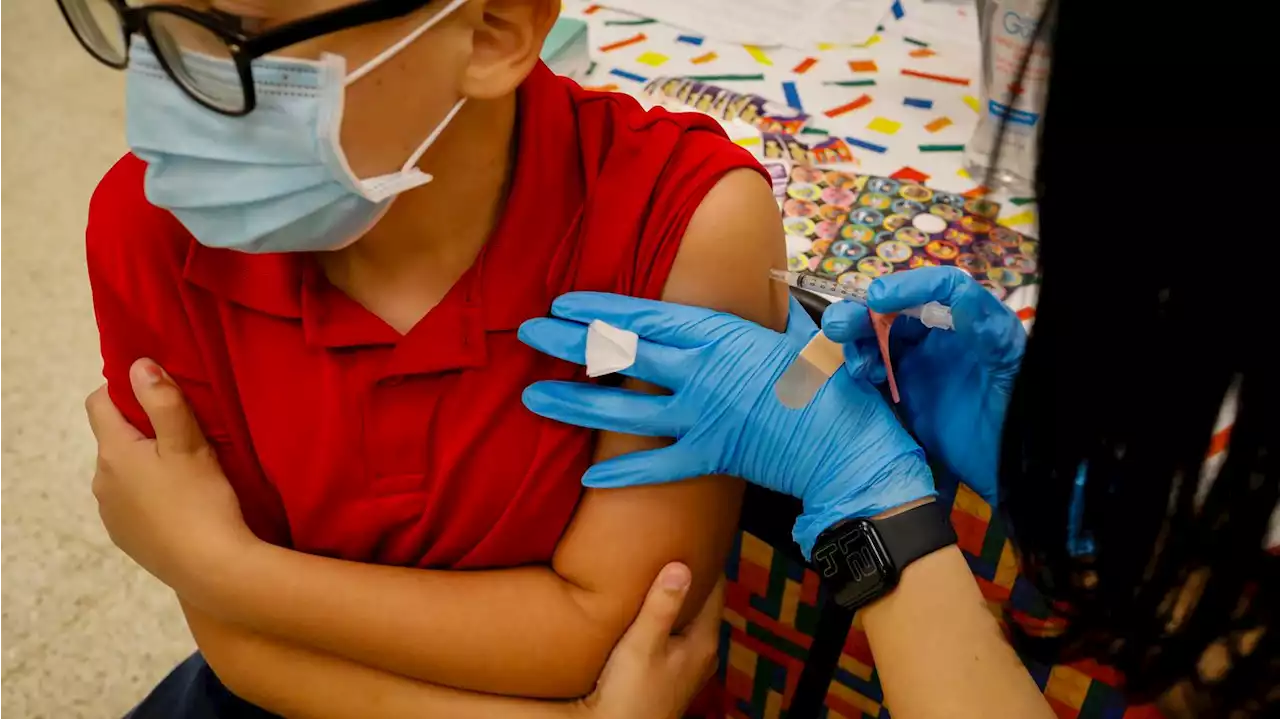 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Weiterlesen »
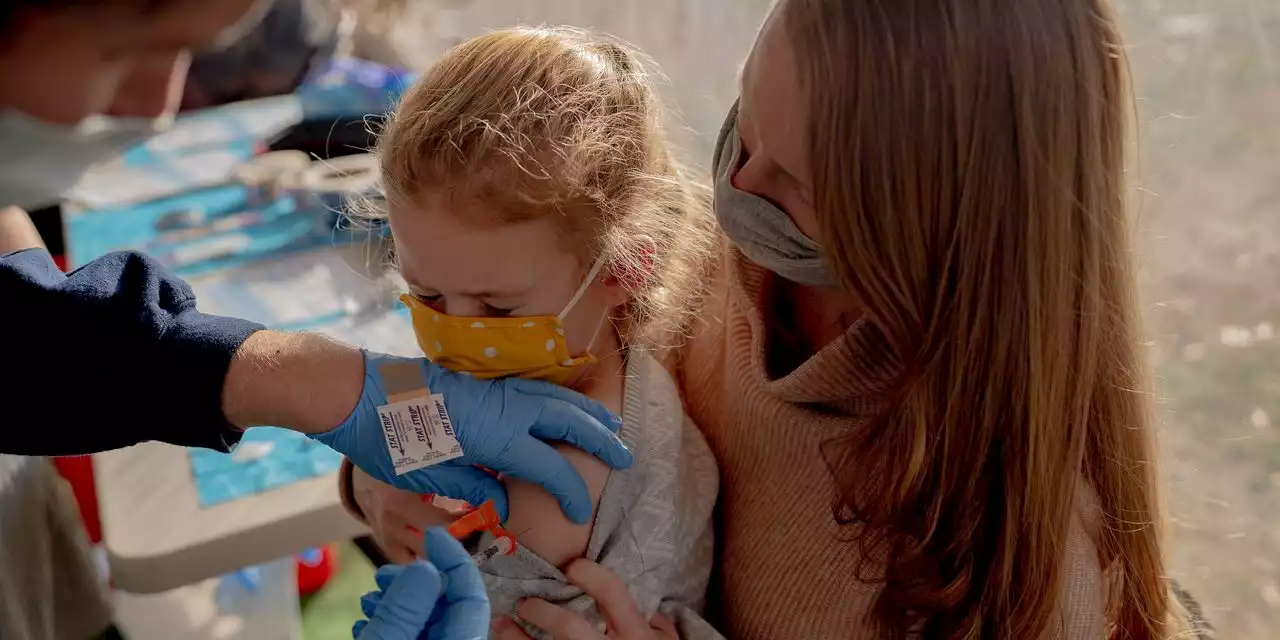 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Weiterlesen »
