The Food and Drug Administration announced Monday it has approved a new drug to prevent RSV in babies and toddlers for the first time.
The Food and Drug Administration announced Monday it has approved a new drug to prevent RSV in babies and toddlers for the first time.
"RSV can cause serious disease in infants and some children and results in a large number of emergency department and physician office visits each year," said John Farley, director of the Office of Infectious Diseases in the FDA's Center for Drug Evaluation and Research."Today’s approval addresses the great need for products to help reduce the impact of RSV disease on children, families and the health care system.
According to the Centers for Disease Control and Prevention, RSV causes 58,000 hospitalizations annually among children under age 5. Early symptoms tend to include a runny nose, decrease in appetite, and cough. Those symptoms can worsen, causing inflammation of the small airways in the lung.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
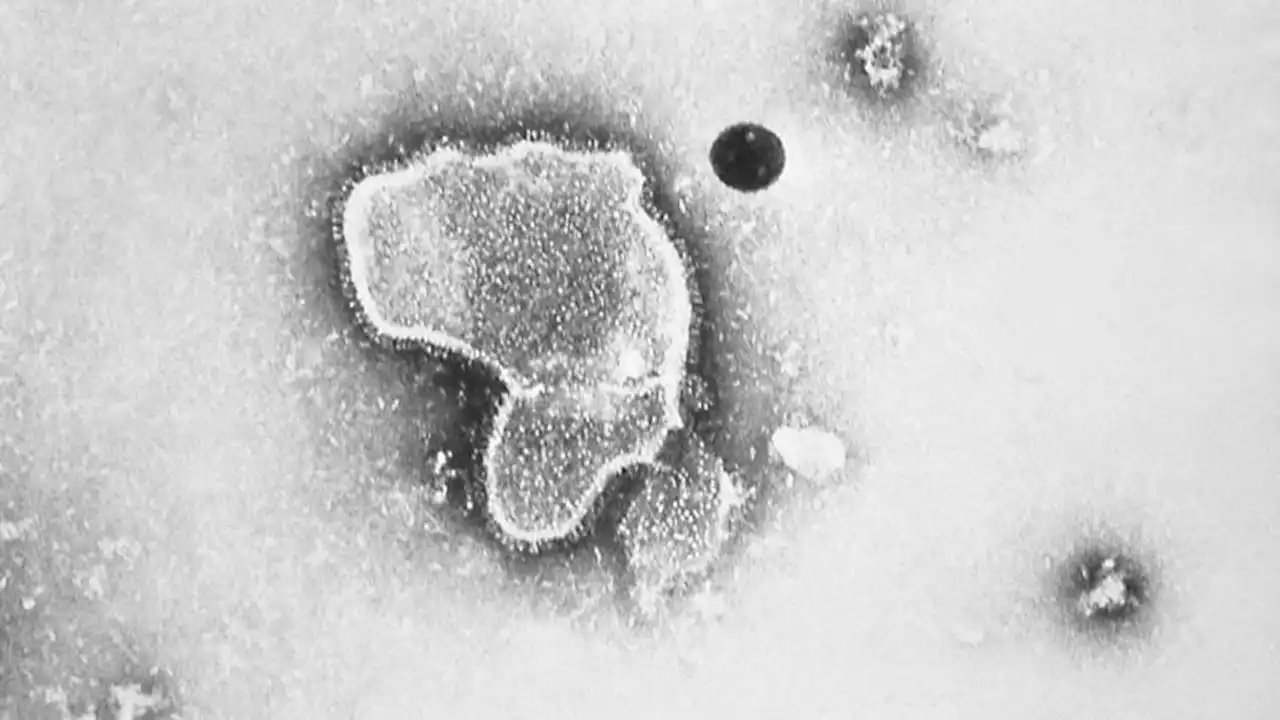 FDA approves antibody to protect infants from RSV | CNNParents and pediatricians will have a new option this fall to protect babies from a lung-attacking virus that is the leading cause of hospitalization in infants under a year of age in the United States every year.
FDA approves antibody to protect infants from RSV | CNNParents and pediatricians will have a new option this fall to protect babies from a lung-attacking virus that is the leading cause of hospitalization in infants under a year of age in the United States every year.
Weiterlesen »
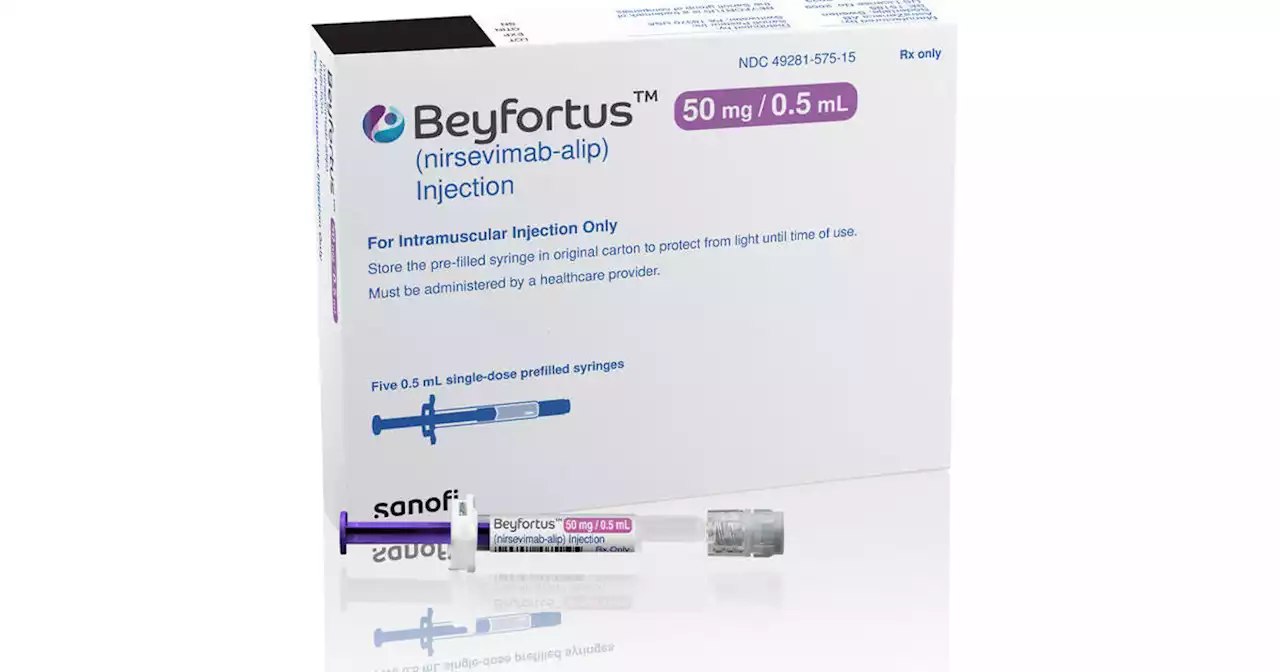 FDA approves new drug to protect babies from RSVThe FDA approved a new drug to protect babies from RSV, the leading cause of hospitalization for U.S. infants.
FDA approves new drug to protect babies from RSVThe FDA approved a new drug to protect babies from RSV, the leading cause of hospitalization for U.S. infants.
Weiterlesen »
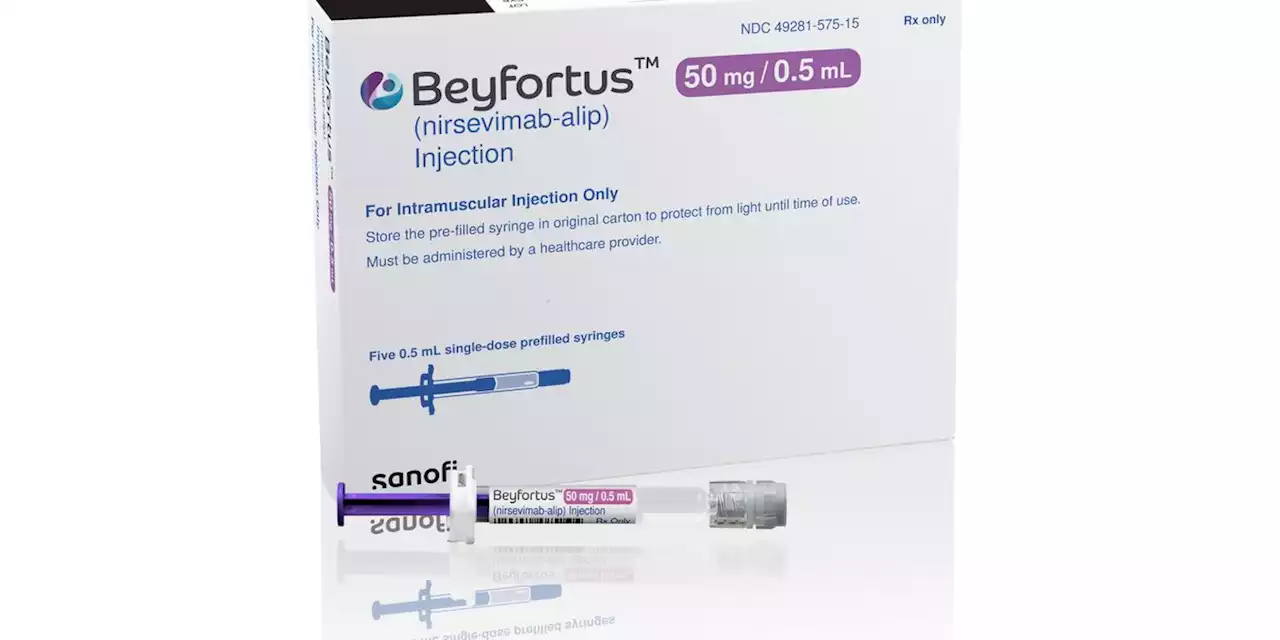 New drug to protect babies and toddlers from RSV gets FDA approval ahead of cold seasonLast year, a surge in RSV cases flooded U.S. hospitals with wheezing children. There are no vaccines for babies yet, though Pfizer and other companies are working on them.
New drug to protect babies and toddlers from RSV gets FDA approval ahead of cold seasonLast year, a surge in RSV cases flooded U.S. hospitals with wheezing children. There are no vaccines for babies yet, though Pfizer and other companies are working on them.
Weiterlesen »
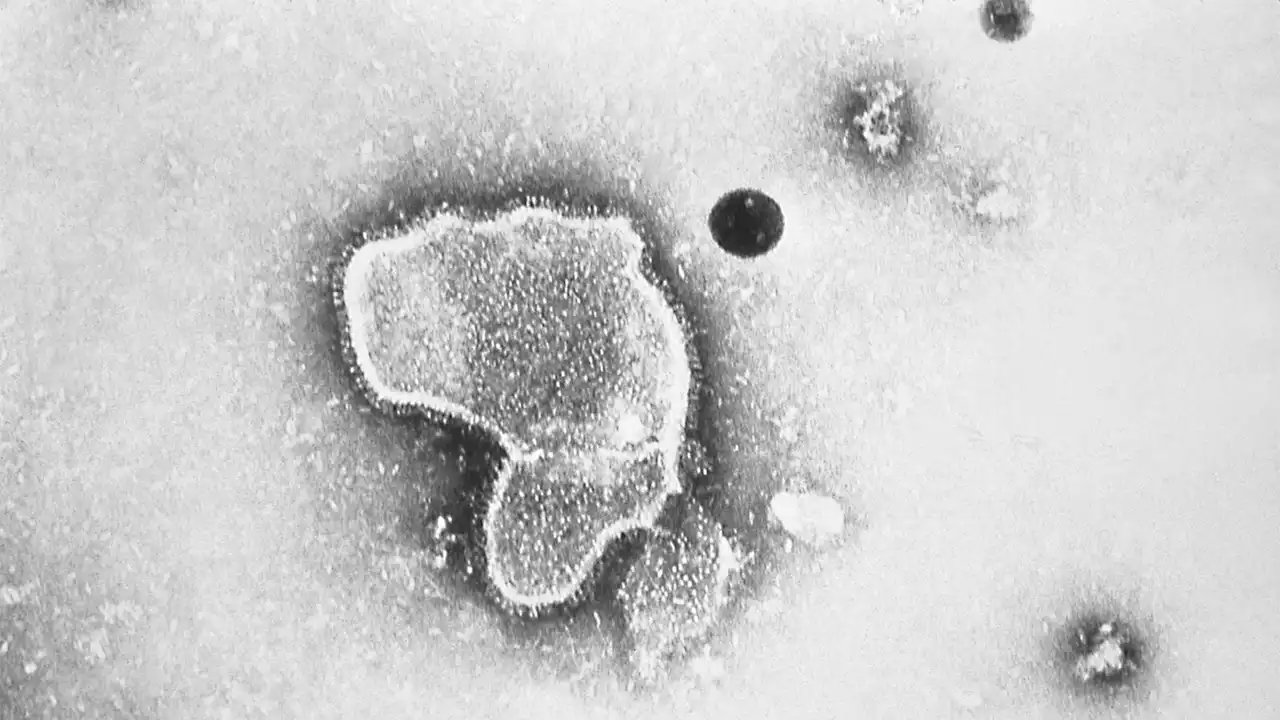 FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
Weiterlesen »
 FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
Weiterlesen »
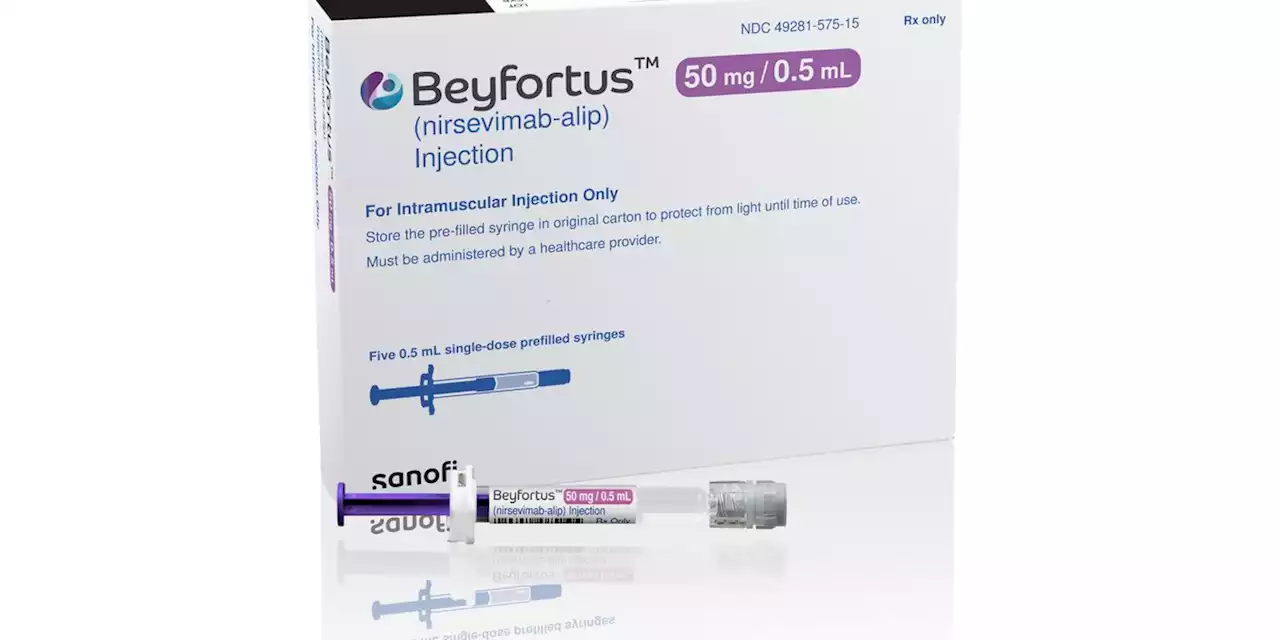 New drug to protect babies and toddlers from RSV gets FDA approval ahead of cold seasonLast year, a surge in RSV cases flooded U.S. hospitals with wheezing children. There are no vaccines for babies yet, though Pfizer and other companies are working on them.
New drug to protect babies and toddlers from RSV gets FDA approval ahead of cold seasonLast year, a surge in RSV cases flooded U.S. hospitals with wheezing children. There are no vaccines for babies yet, though Pfizer and other companies are working on them.
Weiterlesen »
