The FDA approved the gene therapy valoctocogene roxaparvovec for adults with severe hemophilia A.
, uptake in Europe has been delayed due to reimbursement issues, given the cost of treatment and clinical uncertainties.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII. Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 US FDA approves BioMarin's gene therapy for hemophilia AThe U.S. Food and Drug Administration on Thursday approved BioMarin Pharmaceutical's gene therapy for severe hemophilia A, the company said, giving patients with the inherited bleeding disorder an alternative to regular injections of missing blood proteins.
US FDA approves BioMarin's gene therapy for hemophilia AThe U.S. Food and Drug Administration on Thursday approved BioMarin Pharmaceutical's gene therapy for severe hemophilia A, the company said, giving patients with the inherited bleeding disorder an alternative to regular injections of missing blood proteins.
Weiterlesen »
 FDA approves BioMarin’s gene therapy drug for hemophiliaBioMarin Pharmaceutical Inc. received Food and Drug Administration approval of its gene therapy Roctavian to treat hemophilia A, the agency said late...
FDA approves BioMarin’s gene therapy drug for hemophiliaBioMarin Pharmaceutical Inc. received Food and Drug Administration approval of its gene therapy Roctavian to treat hemophilia A, the agency said late...
Weiterlesen »
 FDA approves world's first cellular therapy for type-1 diabetesNamed Lantidra, it's a cellular therapy developed using deceased donor pancreatic cells for the treatment of type 1 diabetes in adults.
FDA approves world's first cellular therapy for type-1 diabetesNamed Lantidra, it's a cellular therapy developed using deceased donor pancreatic cells for the treatment of type 1 diabetes in adults.
Weiterlesen »
 Gene therapy for severe hemophilia is approved by FDAU.S. health regulators have approved a gene therapy for the most common form of hemophilia
Gene therapy for severe hemophilia is approved by FDAU.S. health regulators have approved a gene therapy for the most common form of hemophilia
Weiterlesen »
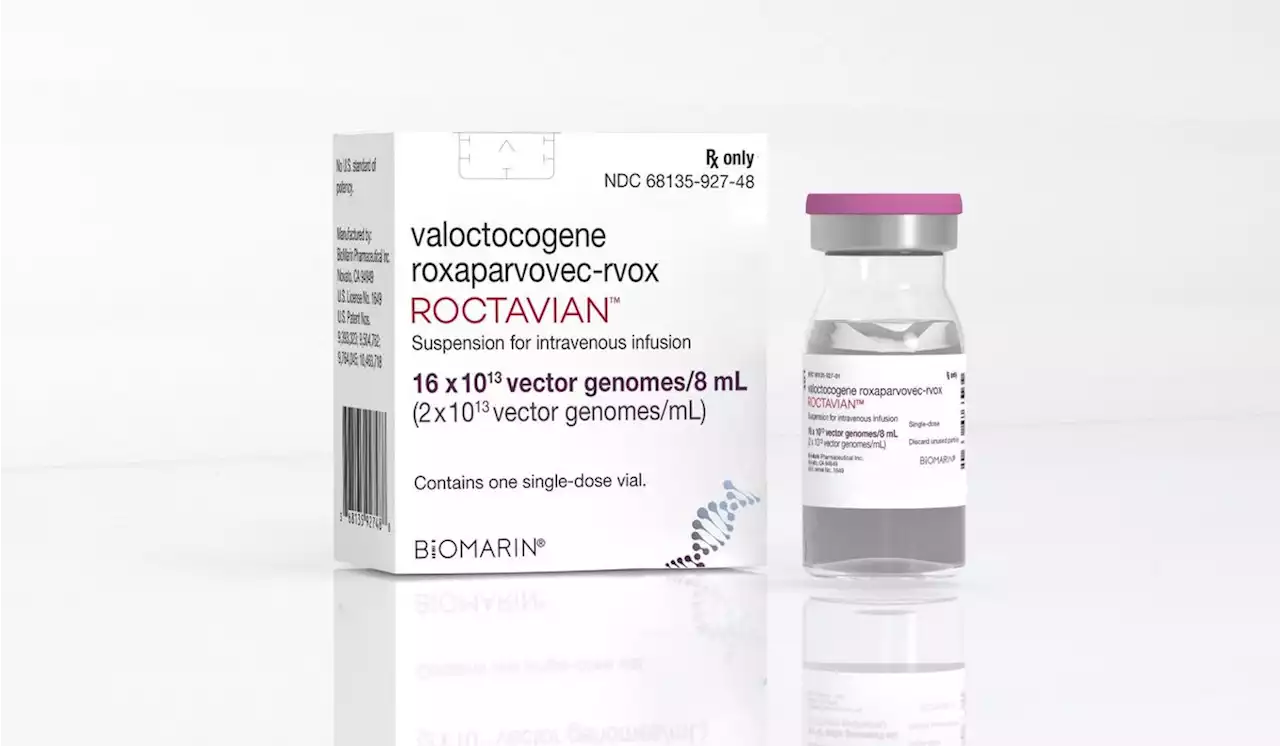 Gene therapy for severe hemophilia is approved by FDAU.S. officials on Thursday approved drugmaker BioMarin's gene therapy for the most common form of hemophilia, an infused treatment that can significantly reduce dangerous bleeding problems.
Gene therapy for severe hemophilia is approved by FDAU.S. officials on Thursday approved drugmaker BioMarin's gene therapy for the most common form of hemophilia, an infused treatment that can significantly reduce dangerous bleeding problems.
Weiterlesen »
 $2.9 million gene therapy for severe hemophilia is approved by FDAU.S. health regulators have approved a gene therapy for the most common form of hemophilia
$2.9 million gene therapy for severe hemophilia is approved by FDAU.S. health regulators have approved a gene therapy for the most common form of hemophilia
Weiterlesen »