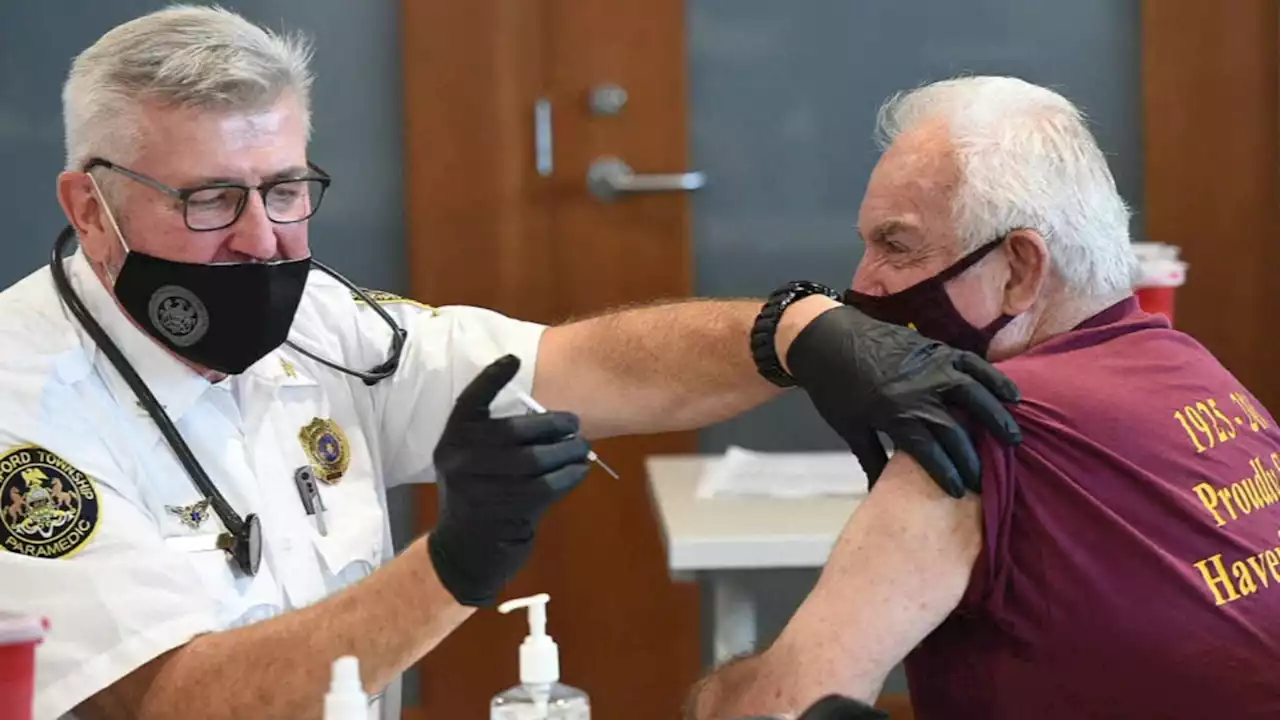
The FDA's decision opens a fourth dose of the Pfizer or Moderna vaccines to that age group at least four months after their previous booster.
WASHINGTON -- U.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds.
Everyone eligible for a first booster who hasn't gotten one yet needs to, FDA vaccine chief Dr. Peter Marks said. But the second booster is only for these higher-risk groups because"current evidence suggests some waning of protection" for them. There's limited evidence to tell how much benefit another booster could offer right now. FDA made the decision without input from its independent panel of experts that has wrestled with how much data is required to expand shots.
During the U.S. omicron wave, two doses were nearly 80% effective against needing a ventilator or death - and a booster pushed that protection to 94%, the CDC recently reported. Vaccine effectiveness was lowest - 74% - in immune-compromised people, the vast majority of whom hadn't gotten a third dose.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
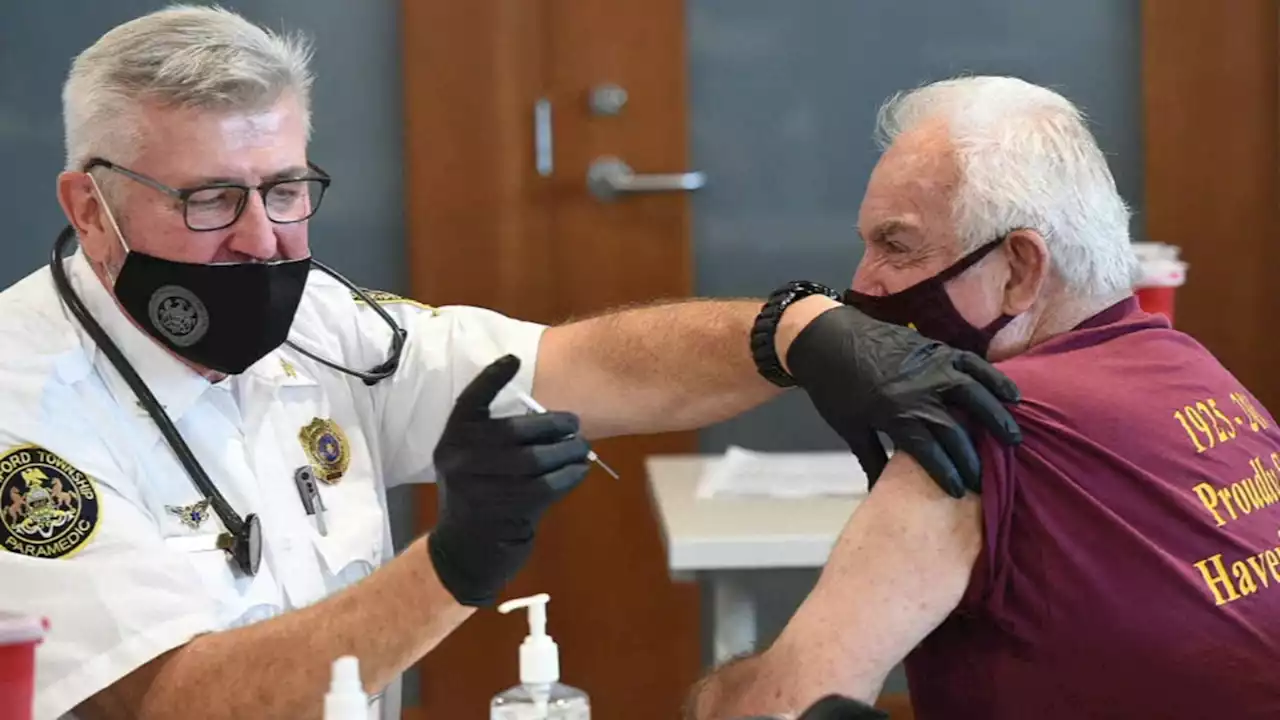 FDA expected to authorize 2nd booster shot for Americans over 50Time for a boost? FDA expected to OK second booster shot for some people. Who may want to get it & who should wait. Now on ABC7
FDA expected to authorize 2nd booster shot for Americans over 50Time for a boost? FDA expected to OK second booster shot for some people. Who may want to get it & who should wait. Now on ABC7
Weiterlesen »
FDA authorizes 2nd booster shot for Americans over 50The decision now moves over to the CDC, where Director Rochelle Walensky will issue instructions on how to implement the authorization.
Weiterlesen »
 The FDA is expected to authorize 2nd boosters for people 50 and upPeople aged 50 and over could soon be eligible for a second Pfizer-BioNTech or Moderna COVID vaccine booster. The administration wants to offer the shots as immunity from the first booster is waning.
The FDA is expected to authorize 2nd boosters for people 50 and upPeople aged 50 and over could soon be eligible for a second Pfizer-BioNTech or Moderna COVID vaccine booster. The administration wants to offer the shots as immunity from the first booster is waning.
Weiterlesen »
 The FDA is expected to authorize 2nd boosters for people 50 and up - Alaska Public MediaAnyone 50 and older could soon be eligible for a second booster dose of the Moderna or Pfizer-BioNtech COVID-19 vaccine. The plan comes as evidence increases that protection from three shots is fading and a fourth shot would help boost immunity back up.
The FDA is expected to authorize 2nd boosters for people 50 and up - Alaska Public MediaAnyone 50 and older could soon be eligible for a second booster dose of the Moderna or Pfizer-BioNtech COVID-19 vaccine. The plan comes as evidence increases that protection from three shots is fading and a fourth shot would help boost immunity back up.
Weiterlesen »
 The FDA is expected to authorize 2nd boosters for people 50 and upAnyone 50 years and older could soon be eligible for a second booster dose of the Moderna or Pfizer COVID-19 vaccine. The plan comes as evidence increases that protection from three shots is fading and a fourth shot would help boost immunity back up.
The FDA is expected to authorize 2nd boosters for people 50 and upAnyone 50 years and older could soon be eligible for a second booster dose of the Moderna or Pfizer COVID-19 vaccine. The plan comes as evidence increases that protection from three shots is fading and a fourth shot would help boost immunity back up.
Weiterlesen »
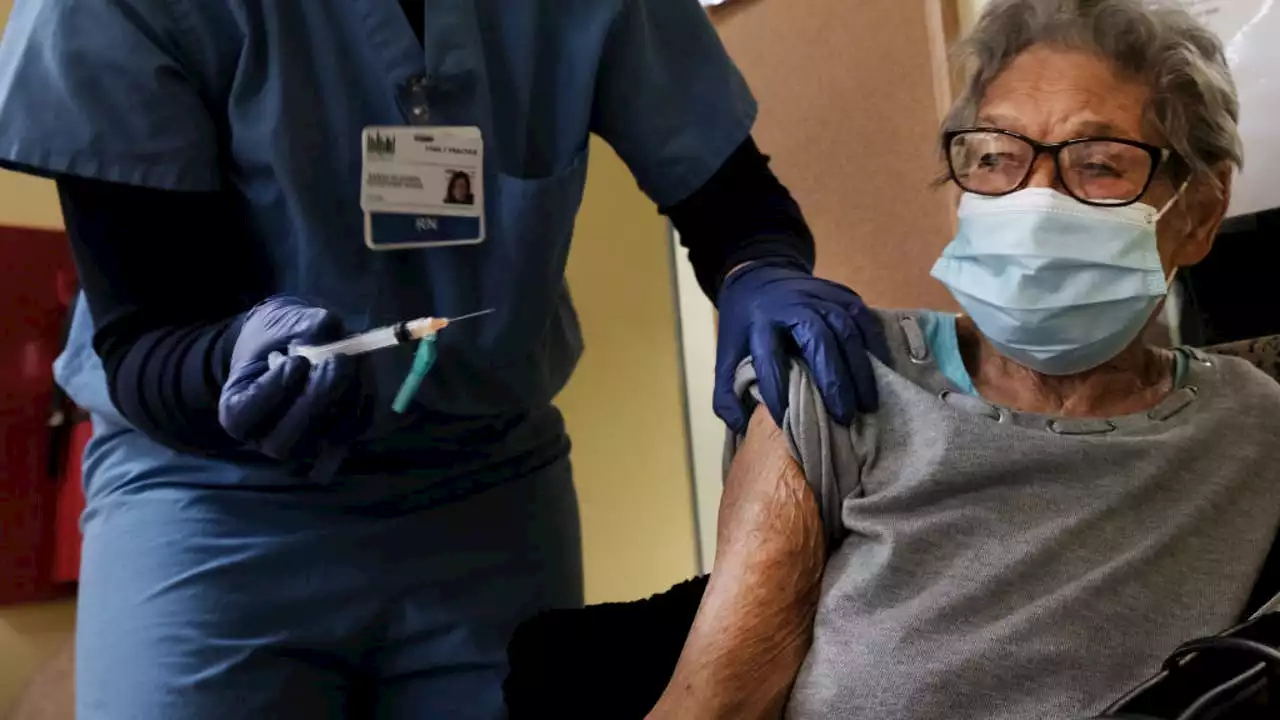 FDA approves 2nd COVID-19 vaccine booster for those 50 and olderU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
FDA approves 2nd COVID-19 vaccine booster for those 50 and olderU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
Weiterlesen »