A new monoclonal antibody drug has proved to be effective against the coronavirus omicron variant and omicron subvariant, according to the Food and Drug Administration.
The agency has given emergency use authorization for the drug to be used as a treatment option for those 12 and older and weighing at least 88 pounds with “mild to moderate COVID-19.” It’s “not authorized for patients who are hospitalized due to COVID-19 or require oxygen therapy due to COVID-19,” the FDA says.
“Today’s action makes available another monoclonal antibody that shows activity against omicron, at a time when we are seeking to further increase supply,” said Dr. Patrizia Cavazzoni, the director of the FDA’s Center for Drug Evaluation and Research, in a statement. The new authorization comes after Eli Lilly’s other monoclonal antibody treatment — bamlanivimab and etesevimab — was restricted for use by the FDA and found to be “highly unlikely to be active against the omicron variant” on Jan. 24, McClatchy News reported. The same happened for Regeneron’s monoclonal antibody treatment, REGEN-COV .
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 U.S. FDA advisers call for new trial of Lilly, Innovent lung cancer drugInnovent Biologics Inc and Eli Lilly and Co should be required to conduct a trial of their lung cancer drug that is applicable to the U.S. population, a panel of advisers to the U.S. Food and Drug Administration recommended Thursday.
U.S. FDA advisers call for new trial of Lilly, Innovent lung cancer drugInnovent Biologics Inc and Eli Lilly and Co should be required to conduct a trial of their lung cancer drug that is applicable to the U.S. population, a panel of advisers to the U.S. Food and Drug Administration recommended Thursday.
Weiterlesen »
 FDA authorizes new Covid antibody drug to fight omicron variantThe FDA has cleared a new antibody drug, that targets the omicron variant, for adults and adolescent patients with mild-to-moderate cases.
FDA authorizes new Covid antibody drug to fight omicron variantThe FDA has cleared a new antibody drug, that targets the omicron variant, for adults and adolescent patients with mild-to-moderate cases.
Weiterlesen »
 FDA Authorizes Use of New Eli Lilly Covid-19 Antibody TreatmentThe FDA authorized the use of a new Covid-19 antibody drug from Eli Lilly that retains effectiveness against the Omicron variant, filling a void after authorities stopped distributing some older antibody drugs that lost effectiveness against the strain
FDA Authorizes Use of New Eli Lilly Covid-19 Antibody TreatmentThe FDA authorized the use of a new Covid-19 antibody drug from Eli Lilly that retains effectiveness against the Omicron variant, filling a void after authorities stopped distributing some older antibody drugs that lost effectiveness against the strain
Weiterlesen »
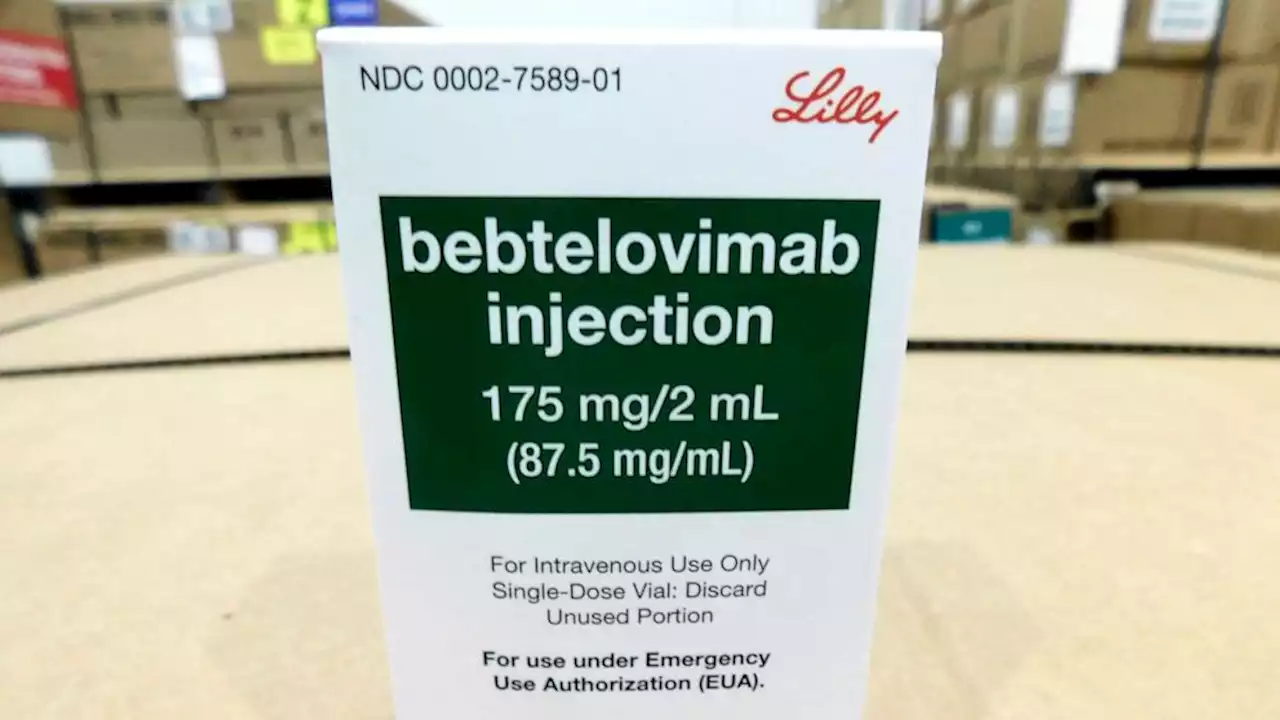 FDA authorizes new monoclonal antibody treatment to fight omicronThe FDA has authorized a new monoclonal antibody treatment for COVID-19, shown to hold up against the omicron variant and BA.2 subvariant.
FDA authorizes new monoclonal antibody treatment to fight omicronThe FDA has authorized a new monoclonal antibody treatment for COVID-19, shown to hold up against the omicron variant and BA.2 subvariant.
Weiterlesen »
 FDA Committee Delays Review of Pfizer-BioNTech's COVID-19 Vaccine for Young ChildrenFDA Committee Delays Review of Pfizer-BioNTech's COVID-19 Vaccine for Young Kids
FDA Committee Delays Review of Pfizer-BioNTech's COVID-19 Vaccine for Young ChildrenFDA Committee Delays Review of Pfizer-BioNTech's COVID-19 Vaccine for Young Kids
Weiterlesen »
