Here's what parents need to know about Pfizer's COVID19 boosters for kids this spring and summer.
With another round of boosters approved for older kids, parents eagerly awaiting vaccine news for their younger children will have to wait a little longer.
As we’ve moved through various stages of the pandemic — with cases inching back up to levels of previous spikes yet there being little meaningful attempts at encouraging masking, social distancing or remote solutions — parents are understandably eager to ensure their children are as protected as possible as they move about their lives. On Tuesday, the
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA Clears COVID Booster Shot For Healthy Kids Ages 5 To 11U.S. regulators hope an extra vaccine dose, which still needs the CDC's approval, will enhance children's protection as infections rise once again.
FDA Clears COVID Booster Shot For Healthy Kids Ages 5 To 11U.S. regulators hope an extra vaccine dose, which still needs the CDC's approval, will enhance children's protection as infections rise once again.
Weiterlesen »
 FDA Limits Use of J&J COVID Vaccine Over Blood Clot RiskThe FDA is limiting who can receive the Johnson & Johnson COVID-19 vaccine because of concerns about the risk of a rare blood clotting condition.
FDA Limits Use of J&J COVID Vaccine Over Blood Clot RiskThe FDA is limiting who can receive the Johnson & Johnson COVID-19 vaccine because of concerns about the risk of a rare blood clotting condition.
Weiterlesen »
 FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
Weiterlesen »
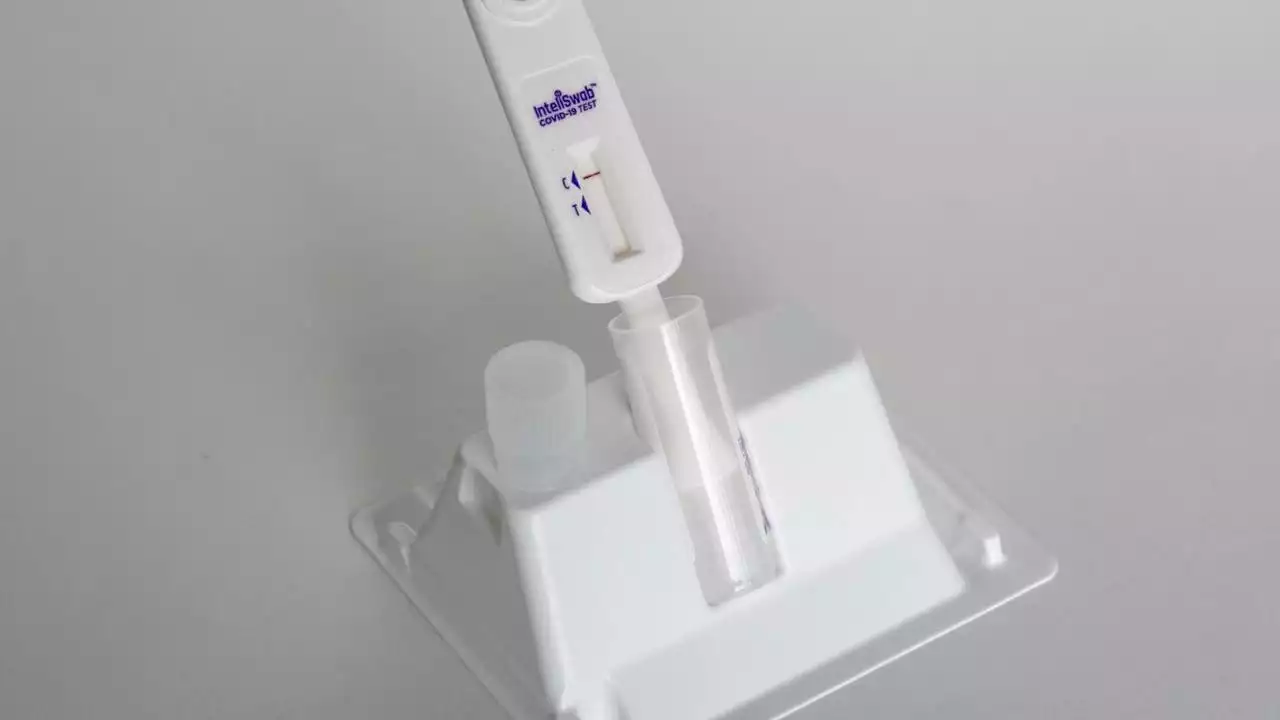 FDA approves first non-prescription COVID test that also detects flu, RSVThe U.S. Food and Drug Administration issued its approval Monday of the first non-prescription at-home COVID-19 test that also detects the flu.
FDA approves first non-prescription COVID test that also detects flu, RSVThe U.S. Food and Drug Administration issued its approval Monday of the first non-prescription at-home COVID-19 test that also detects the flu.
Weiterlesen »
 FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
Weiterlesen »