The FDA has approved empagliflozin and empagliflozin combined with metformin for the treatment of type 2 diabetes in children aged 10 years and older.
At week 26, the children treated with empagliflozin showed an average 0.2 percentage point decrease in A1c, compared with a 0.7-point increase among those taking placebo. Use of empagliflozin was also associated with lower fasting plasma glucose levels compared to placebo., regardless of other glucose-lowering therapies that were being taken.
Reduction in A1c for participants treated with linagliptin was not statistically significant in comparison with placebo. There was a numerical reduction of 0.34% . "Across the lifespan, we know that people living with type 2 diabetes have a high risk for many diabetes complications, so it's important to recognize and treat diabetes early in its course," said Lori Laffel, MD, lead investigator of the DINAMO study, in a"These findings are particularly important given the need for more therapeutic options, especially oral agents, to manage type 2 diabetes in young people as, to date, metformin [has been] the only globally available oral...
Miriam E. Tucker is a freelance journalist based in the Washington DC area. She is a regular contributor to Medscape, with other work appearing in the Washington Post, NPR's Shots blog, and Diabetes Forecast magazine. She is on Twitter @MiriamETucker.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA OKs Drug for Cholestatic Pruritus in Alagille SyndromeOdevixibat is indicated for treatment of cholestatic pruritus associated with Alagille syndrome in patients as young as 12 months. It marks the second indication for the drug.
FDA OKs Drug for Cholestatic Pruritus in Alagille SyndromeOdevixibat is indicated for treatment of cholestatic pruritus associated with Alagille syndrome in patients as young as 12 months. It marks the second indication for the drug.
Weiterlesen »
 FDA OKs Low-Dose Colchicine for Broad CV IndicationThe drug represents the first specific anti-inflammatory drug for the reduction of cardiovascular events for patients with established atherosclerotic disease or with multiple risk factors.
FDA OKs Low-Dose Colchicine for Broad CV IndicationThe drug represents the first specific anti-inflammatory drug for the reduction of cardiovascular events for patients with established atherosclerotic disease or with multiple risk factors.
Weiterlesen »
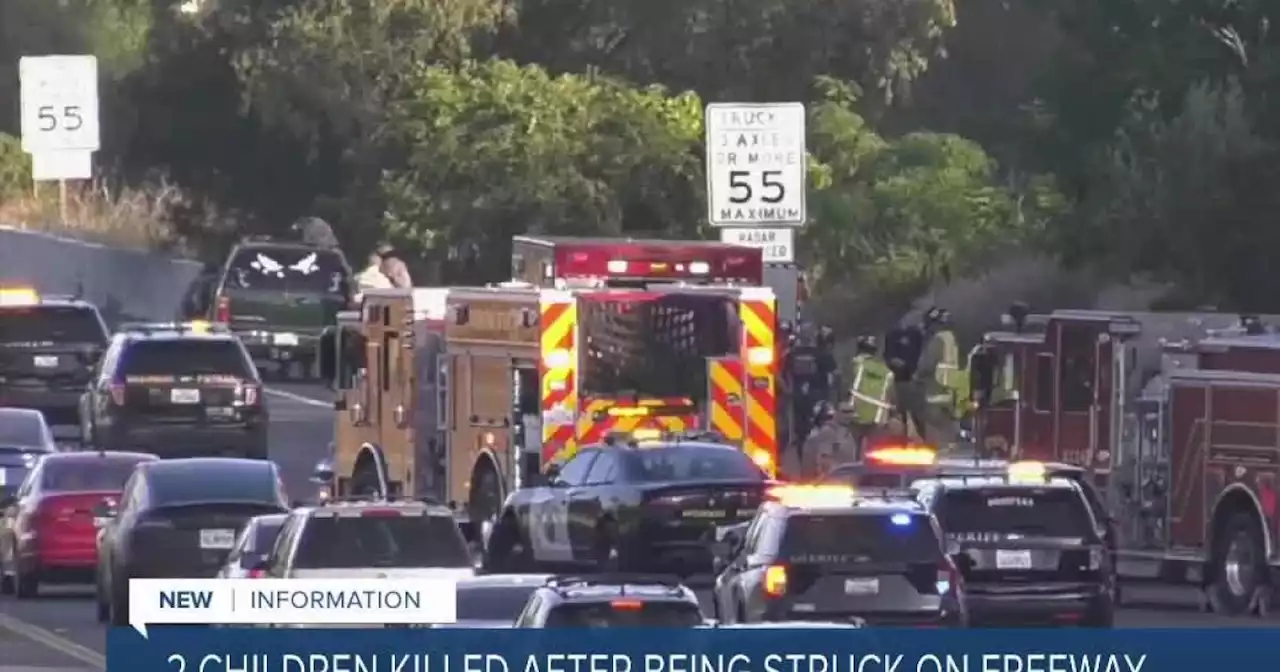 2 children hit by car, killed on SR-78 in Vista; children's mother arrestedThe children were retrieving luggage that fell from their mother's vehicle when they were struck by an oncoming car, Vista Fire Department officials said.
2 children hit by car, killed on SR-78 in Vista; children's mother arrestedThe children were retrieving luggage that fell from their mother's vehicle when they were struck by an oncoming car, Vista Fire Department officials said.
Weiterlesen »
School closure study, discussion and decisions: In 5-2 vote, SAISD board OKs painful processWith too many schools for a shrinking number of students, SAISD trustees set sights on a final 'rightsizing' proposal by Nov. 13.
Weiterlesen »
 FDA Warns of Tattoo Ink Tied to Dangerous InfectionsThe FDA released the new draft guidance aiming to reduce the use of pathogen-contaminated tattoo ink, which can cause stubborn infections that are especially hard to treat, dermatologists said.
FDA Warns of Tattoo Ink Tied to Dangerous InfectionsThe FDA released the new draft guidance aiming to reduce the use of pathogen-contaminated tattoo ink, which can cause stubborn infections that are especially hard to treat, dermatologists said.
Weiterlesen »
 Report: U.S. 'Outlier' Among Western Nations in Transgender 'Care' for ChildrenThe U.S. is an 'outlier' among Western nations in providing body-altering drugs and surgery to children who identify as transgender.
Report: U.S. 'Outlier' Among Western Nations in Transgender 'Care' for ChildrenThe U.S. is an 'outlier' among Western nations in providing body-altering drugs and surgery to children who identify as transgender.
Weiterlesen »
