The Food and Drug Administration on Thursday unveiled its proposed ban on menthol cigarettes and flavored cigars, a move that health experts say could save hundreds of thousands of lives.
PUBLISHED 12:30 PM EDT Apr. 28, 2022The Food and Drug Administration on Thursday unveiled its proposed ban on menthol cigarettes and flavored cigars
Studies shared by the FDA indicate a 15% reduction in smoking over 40 years if menthol cigarettes were no longer available for sale in the U.S., and 324,000 to 654,000 smoking deaths would be avoided over the same time period – including 92,000 to 238,000 among African Americans "Through careful consideration of the scientific evidence and our authorities under the Tobacco Control Act, we've determined that these actions are appropriate for protection of the public health," FDA Commissioner Dr. Robert Califf told a Senate subcommittee on Thursday.
“The proposed rules would help prevent children from becoming the next generation of smokers and help adult smokers quit,” Health and Human Services Secretary Xavier Becerra said in a statement. “Additionally, the proposed rules represent an important step to advance health equity by significantly reducing tobacco-related health disparities.”
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Inside the Gates: Mental health care centers call for early interaction on military children’s mental healthThe average military child changes school six to nine times before they graduate high school, according to MaryBeth Goodman, the clinic director at the Steven A. Cohen Military Family Clinic at Alaska Behavioral Health.
Inside the Gates: Mental health care centers call for early interaction on military children’s mental healthThe average military child changes school six to nine times before they graduate high school, according to MaryBeth Goodman, the clinic director at the Steven A. Cohen Military Family Clinic at Alaska Behavioral Health.
Weiterlesen »
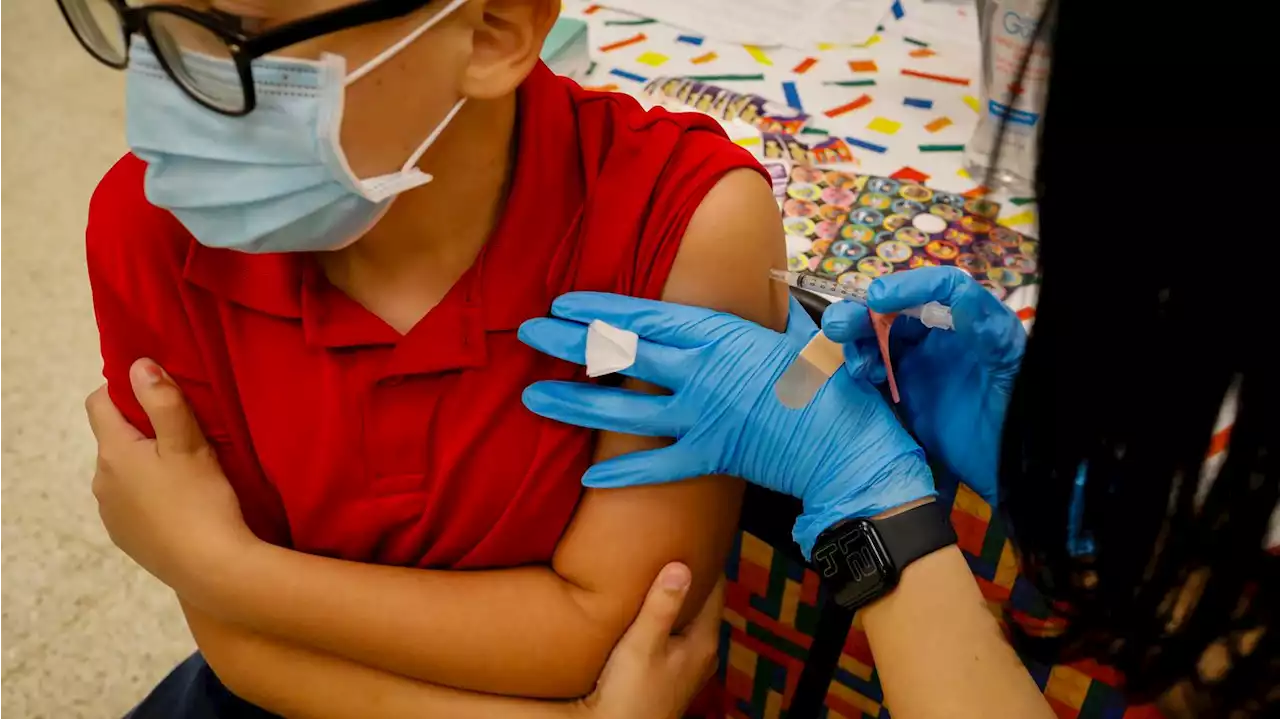 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Weiterlesen »
 Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Weiterlesen »
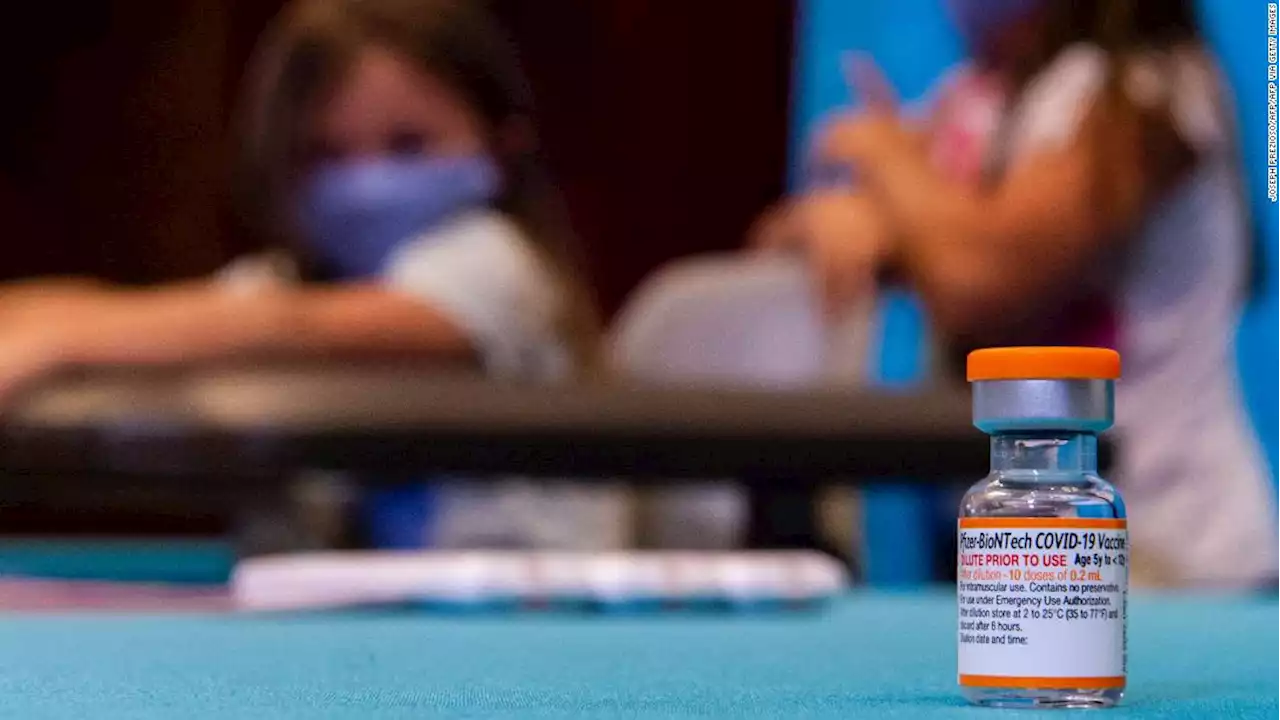 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Weiterlesen »
 FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
Weiterlesen »
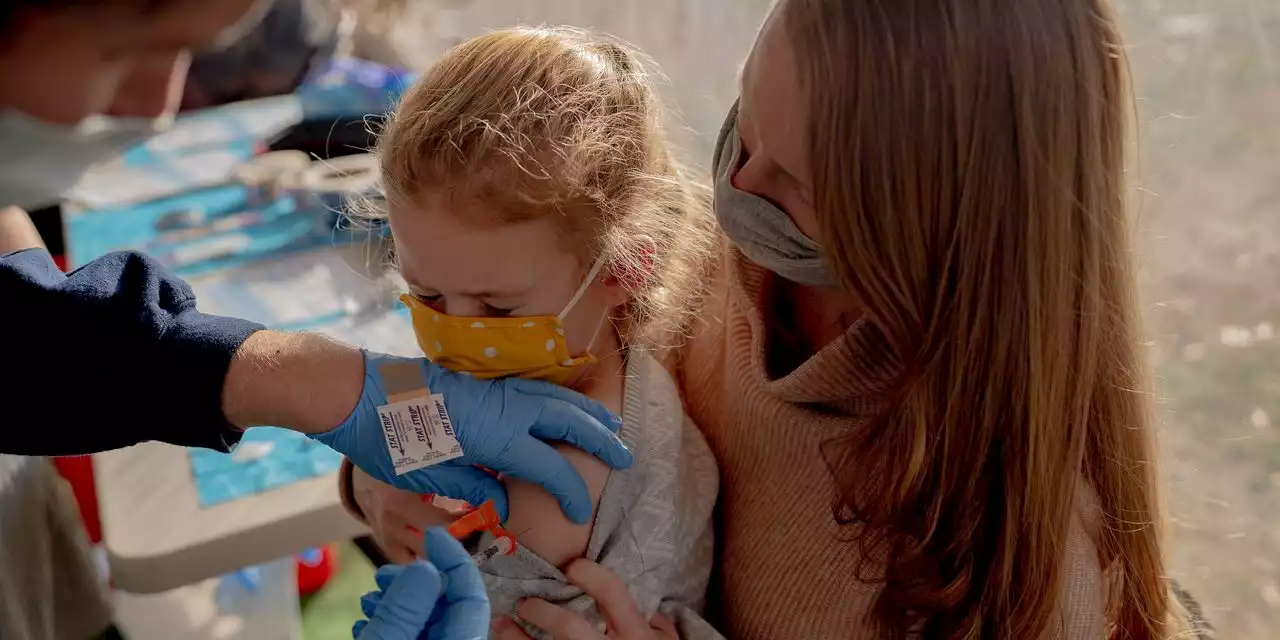 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Weiterlesen »
