The FDA's top vaccine regulator Dr. Peter Marks said shortly after the decision that another round of boosters will likely be needed in the fall.
While some health experts believe the central purposes of the vaccines is to prevent severe illness, others think it is important to also stop infections from the virus. The protection provided by the Pfizer and Moderna vaccines against infection has declined substantially over time, particularly in the context of omicron, which has numerous mutations that give it an enhanced capability to cause breakthrough infections and mild illness.
The Israeli Health Ministry and scientists at Sheba Medical Center found that a fourth dose does restores antibodies that waned off after a third dose among health-care workers ages 18 and older, but it provided little protection against infection. Pfizer cited that study, which has not undergone peer review, among others in its statement on the FDA authorization, focusing on the increased antibodies without highlighting the issues with breakthrough infections.Dr.
While the Ben Gurion study may point in the direction of benefit for older adults at the moment, the evidence for boosting younger adults again is scant as the U.S. considers lowering the eligibility for fourth doses sometime later in the year. Hotez also expressed frustration that the U.S. relies heavily on data from abroad, particularly Israel and the U.K. Offit also questioned why the U.S. is relying on data from countries that are smaller than the U.S. and have different demographic backgrounds.Hildreth said the U.S.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
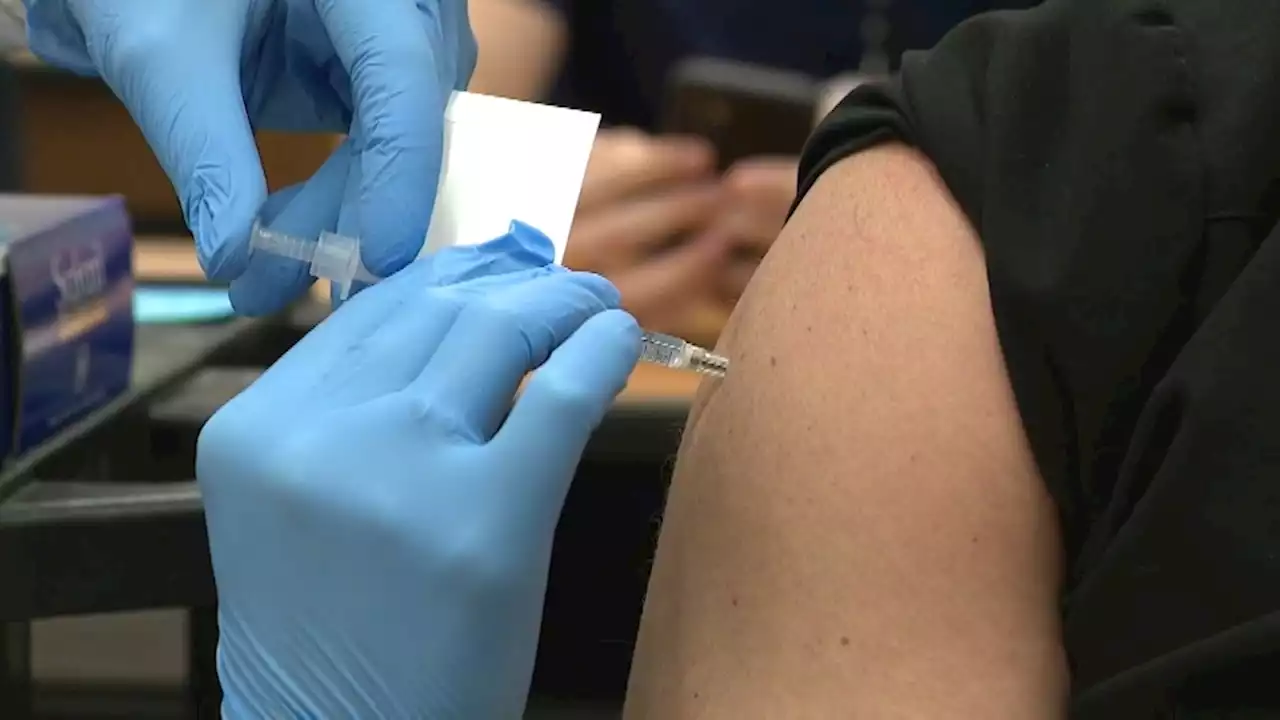 FDA panel to discuss what COVID shots we'll need nextFDA advisers will meet today to discuss what shots we'll need next.
FDA panel to discuss what COVID shots we'll need nextFDA advisers will meet today to discuss what shots we'll need next.
Weiterlesen »
 Watchdog urged to probe McKinsey over work with FDA, opioid manufacturersSenate Democrats are calling on a government watchdog to investigate McKinsey & Company over claims that the consulting giant skirted federal conflict-of-interest rules.
Watchdog urged to probe McKinsey over work with FDA, opioid manufacturersSenate Democrats are calling on a government watchdog to investigate McKinsey & Company over claims that the consulting giant skirted federal conflict-of-interest rules.
Weiterlesen »
 Opinion | FDA Shuts Out Its Own Experts in Authorizing Another Vaccine BoosterFrom WSJopinion: Trust in public health is at an all-time low. When agencies bypass their own experts, it only reinforces the perception that health policy is driven by groupthink and politics, writes Marty Makary.
Opinion | FDA Shuts Out Its Own Experts in Authorizing Another Vaccine BoosterFrom WSJopinion: Trust in public health is at an all-time low. When agencies bypass their own experts, it only reinforces the perception that health policy is driven by groupthink and politics, writes Marty Makary.
Weiterlesen »
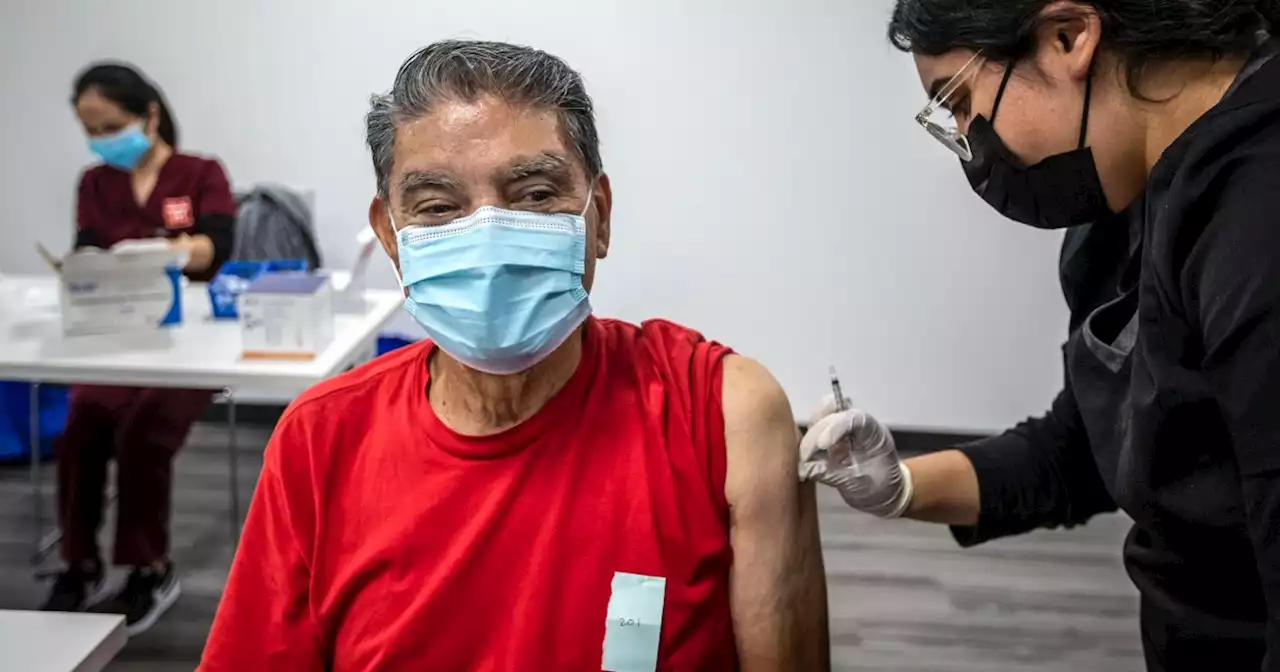 How many Covid shots will U.S. need? FDA panel to discuss what's ahead for fallThe panel meets Wednesday as some experts question whether getting additional doses every few months is a practical public health strategy.
How many Covid shots will U.S. need? FDA panel to discuss what's ahead for fallThe panel meets Wednesday as some experts question whether getting additional doses every few months is a practical public health strategy.
Weiterlesen »
 How Many COVID Shots Will US Need? FDA Panel to Discuss What's Ahead for FallFood and Drug Administration advisers will meet Wednesday to hash out what the future of COVID-19 boosters will look like in the United States.
How Many COVID Shots Will US Need? FDA Panel to Discuss What's Ahead for FallFood and Drug Administration advisers will meet Wednesday to hash out what the future of COVID-19 boosters will look like in the United States.
Weiterlesen »
