It's the second vaccine to receive full FDA approval.
The FDA’s decision to approve Spikevax has come after meticulous rounds of testing. In an ongoing randomized clinical trial , 14,287 vaccine recipients and 14,164 placebo recipients were analyzed. All recipients did not have COVID-19 before receiving their first dose. The trial found that Spikevax was 93% effective in preventing COVID-19. Only 55 cases of COVID-19 were detected in the group who were vaccinated, while 744 COVID-19 cases were detected in the group who received a placebo.
Spikevax was also found to not only be highly effective in preventing COVID-19 but 98% effective in preventing severe illness as a result of the virus., Spikevax can lead to some side effects. The most common side effects include chills, fatigue, nausea, vomiting, swollen lymph nodes, fever, headache, muscle or joint pain, and pain, redness, and swelling where the vaccine was injected.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Moderna COVID vaccine gets full FDA approvalThe shot has been given to tens of millions of Americans since its emergency authorization over a year ago.
Moderna COVID vaccine gets full FDA approvalThe shot has been given to tens of millions of Americans since its emergency authorization over a year ago.
Weiterlesen »
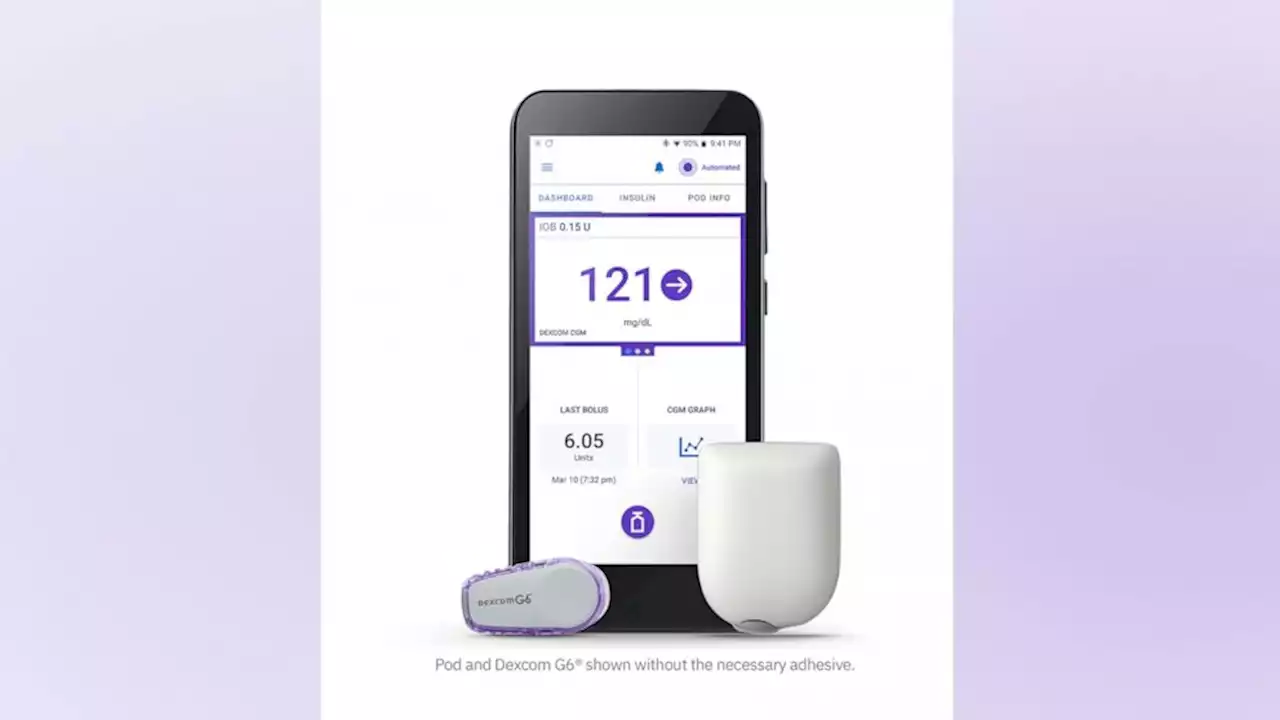 FDA approves 1st automated, tubeless insulin pump for people with Type 1 diabetesNew technology for people with Type 1 diabetes, one that has been nearly a decade in the making, approved by the FDA.
FDA approves 1st automated, tubeless insulin pump for people with Type 1 diabetesNew technology for people with Type 1 diabetes, one that has been nearly a decade in the making, approved by the FDA.
Weiterlesen »
 Moderna's COVID-19 vaccine gets full FDA approvalModerna's COVID-19 vaccine called “Spikevax” has been given full approval from the U.S. Food and Drug Administration.
Moderna's COVID-19 vaccine gets full FDA approvalModerna's COVID-19 vaccine called “Spikevax” has been given full approval from the U.S. Food and Drug Administration.
Weiterlesen »
