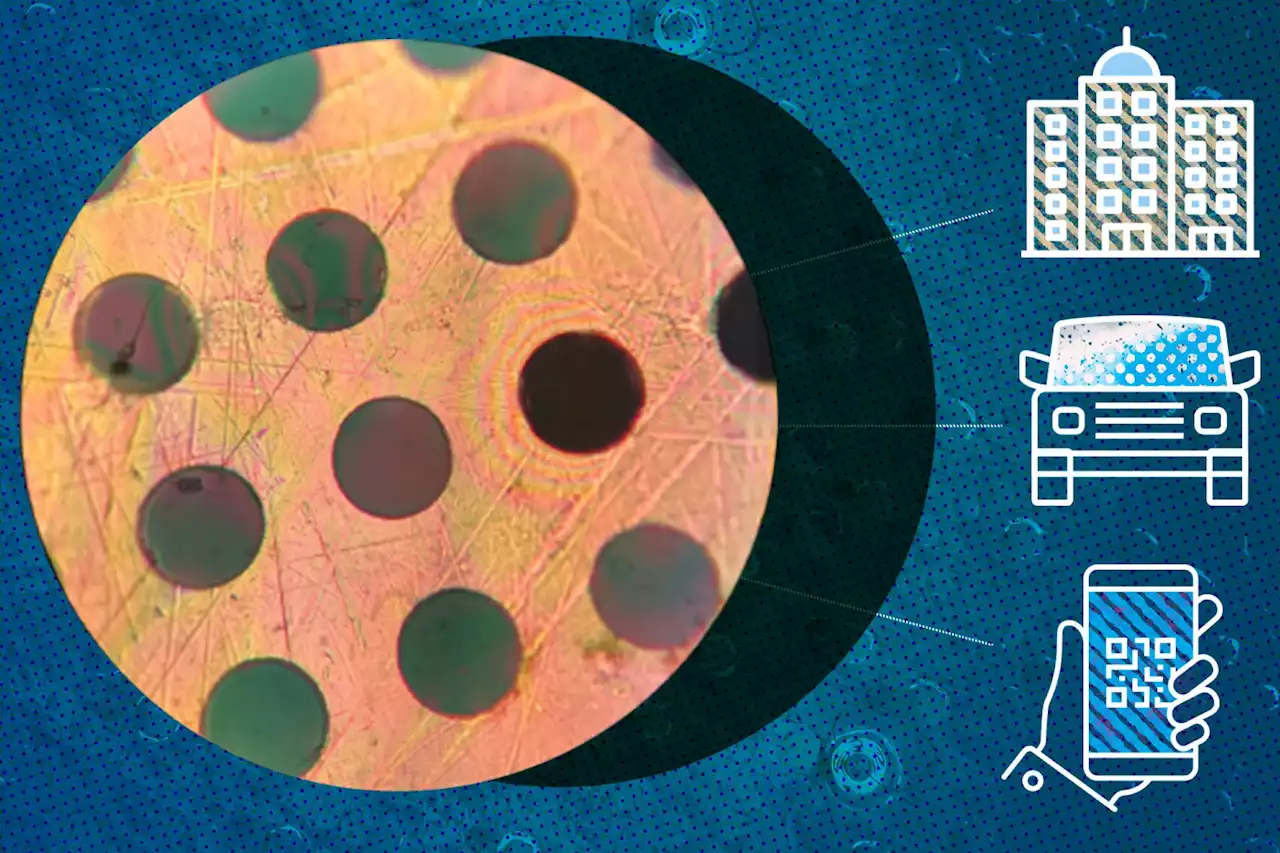
A new polymer synthesis mechanism opens doors to transform common plastics into membrane form.
Scientists have created the plastic equivalent of steel—with all its strength but none of its heft. Plastics, which chemists sometimes call polymers, are a class of long-chained molecules
“They stick like Velcro to each other,” says lead author Michael Strano, a chemical engineer at the Massachusetts Institute of Technology. Ripping the material not only requires snapping the individual molecular strands, but also overcoming the vast intermolecular hydrogen bonds that pervade across the entire polymer bundle.
“Can you polymerize in a sheet? It turns out that under most circumstances, until our work, you could not,” says Strano. “So, we found a new mechanism.” In this recent work, his team overcame the hurdles to make this two-dimensional polymerization possible. To quantify the mechanical properties of the polymer material, the researchers measured the necessary force to poke holes in suspended sheets of the material with a fine needle. The polyamide was indeed stiffer than conventional polymers such as nylon, the fabric used to make parachutes. Notably, wrenching this ultra-strong polyamide apart required two times more force than breaking steel of the same thickness.