FDA OKs RSV vaccine for infants
The US Food and Drug Administration on Monday approved a vaccine against respiratory syncytial virus for newborns and infants.
The monoclonal antibody Beyfortus , which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.under age 5 years are hospitalized with an RSV infection annually in the United States.
Sanofi and AstraZeneca, which jointly developed the vaccine, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection. Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the vaccine reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% CI, 50.6 - 87.3;
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
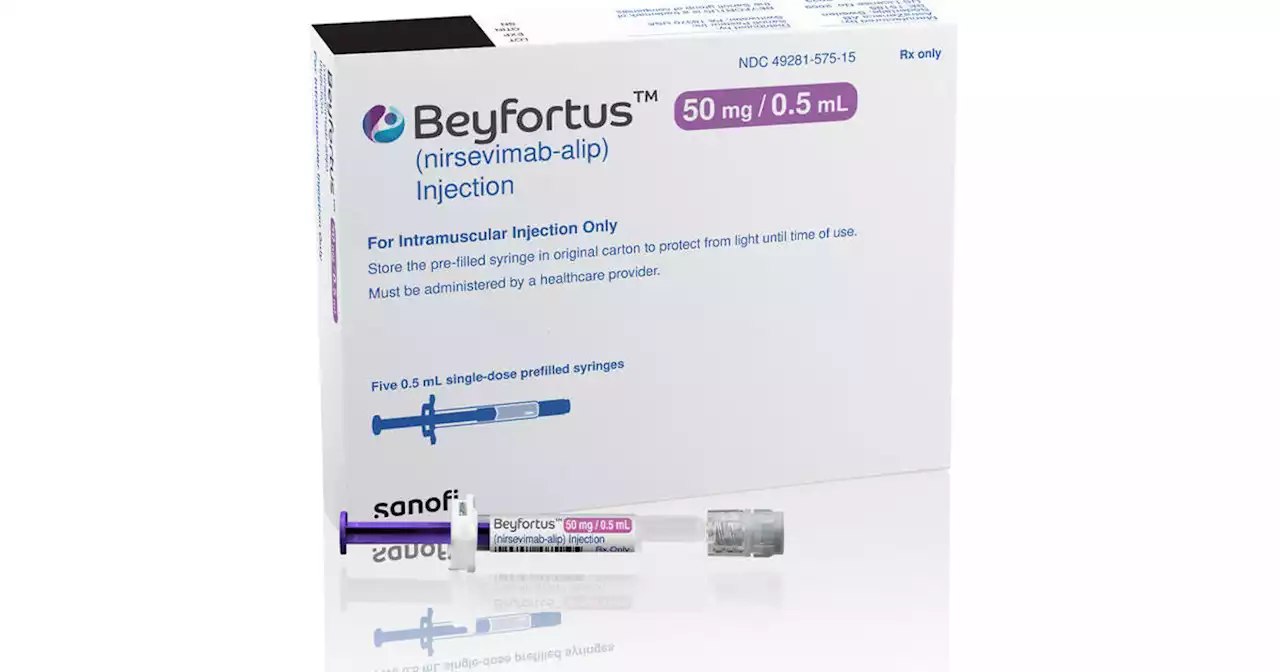 FDA approves new drug to protect babies from RSVThe FDA approved a new drug to protect babies from RSV, the leading cause of hospitalization for U.S. infants.
FDA approves new drug to protect babies from RSVThe FDA approved a new drug to protect babies from RSV, the leading cause of hospitalization for U.S. infants.
Weiterlesen »
 FDA approves antibody to protect infants from RSV | CNNParents and pediatricians will have a new option this fall to protect babies from a lung-attacking virus that is the leading cause of hospitalization in infants under a year of age in the United States every year.
FDA approves antibody to protect infants from RSV | CNNParents and pediatricians will have a new option this fall to protect babies from a lung-attacking virus that is the leading cause of hospitalization in infants under a year of age in the United States every year.
Weiterlesen »
 FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
Weiterlesen »
 FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
Weiterlesen »
 FDA approves drug to prevent RSV for young childrenThe Food and Drug Administration announced Monday it has approved a new drug to prevent RSV in babies and toddlers for the first time.
FDA approves drug to prevent RSV for young childrenThe Food and Drug Administration announced Monday it has approved a new drug to prevent RSV in babies and toddlers for the first time.
Weiterlesen »
 FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
Weiterlesen »
