ICYM the latest on getting to a Covid vaccine for kids under 5 👇
But FDA asked them last month to begin submitting data to support emergency use authorization for the first two shots in the expected three-dose series as the Omicron variant sickened more children than at any other point in the pandemic.
The regulators suggested the interim data was insufficient to begin considering authorization of the vaccine before the trial of three doses is complete. Pfizer and BioNTech said in a statement they expect data on the three-dose regimen by early April. In a call with reporters, Marks did not say when the vaccine for children under 5 would be considered, but that FDA would act quickly once it has sufficient data. He repeatedly declined to describe specific data that spurred the agency’s decision to postpone the meeting.
He added that it’s “probably a safe assumption” that, by waiting for more data, FDA will be able to consider an emergency use authorization for the youngest children based on data from actual infections, rather than just measuring antibody levels and comparing them to older kids.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA postpones advisory panel meeting on Pfizer Covid-19 vaccine for younger childrenThe US Food and Drug Administration is postponing an advisory panel meeting on authorizing Pfizer’s Covid-19 vaccine in children under 5.
FDA postpones advisory panel meeting on Pfizer Covid-19 vaccine for younger childrenThe US Food and Drug Administration is postponing an advisory panel meeting on authorizing Pfizer’s Covid-19 vaccine in children under 5.
Weiterlesen »
 Pfizer postpones FDA request for Covid vaccine for kids under 5The company said it believes three doses 'may provide a higher level of protection in this age group.'
Pfizer postpones FDA request for Covid vaccine for kids under 5The company said it believes three doses 'may provide a higher level of protection in this age group.'
Weiterlesen »
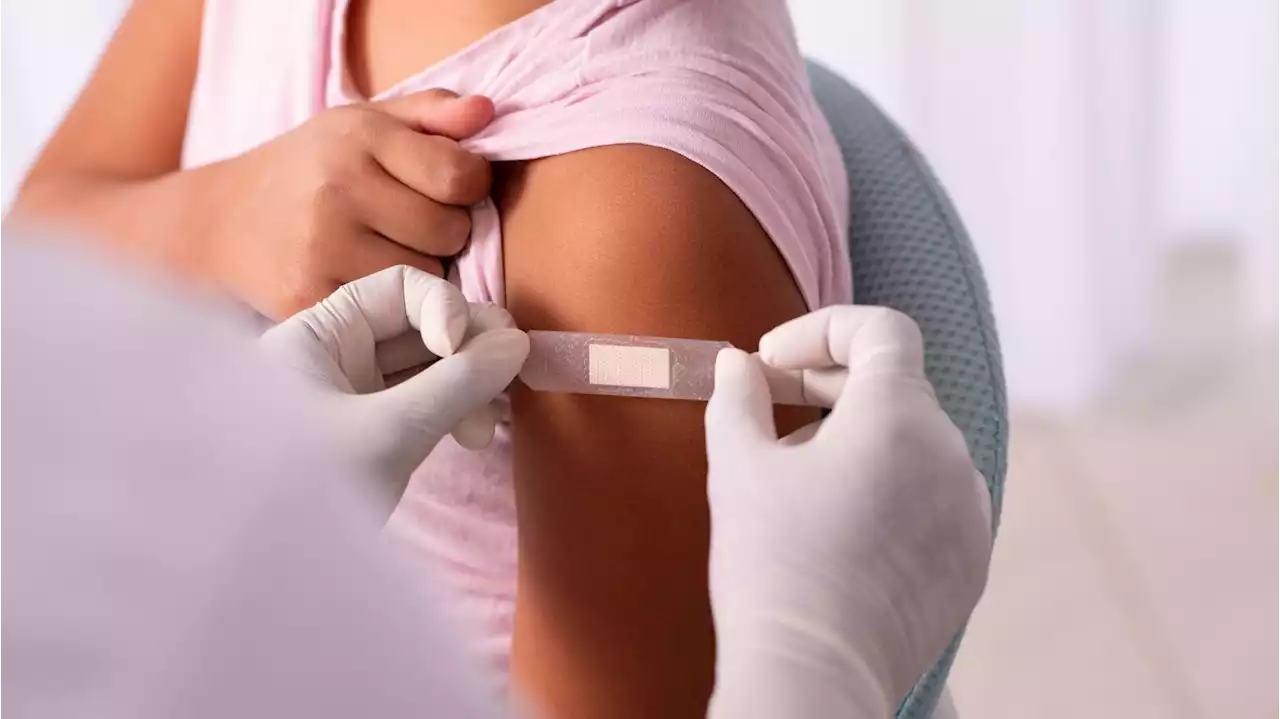 Pfizer Postpones Request To Give COVID Vaccines To Kids Under 5The company said it will wait until it has more data on a third dose.
Pfizer Postpones Request To Give COVID Vaccines To Kids Under 5The company said it will wait until it has more data on a third dose.
Weiterlesen »
 Covid-19 vaccine authorization for younger children delayed as FDA postpones meetingThe US Food and Drug Administration is postponing an advisory panel meeting on authorizing Pfizer’s Covid-19 vaccine in children under 5.
Covid-19 vaccine authorization for younger children delayed as FDA postpones meetingThe US Food and Drug Administration is postponing an advisory panel meeting on authorizing Pfizer’s Covid-19 vaccine in children under 5.
Weiterlesen »
