US FDA approves Lilly weight-loss drug, will compete with Novo's Wegovy
© Reuters. FILE PHOTO: An Eli Lilly and Company pharmaceutical manufacturing plant is pictured at 50 ImClone Drive in Branchburg, New Jersey, March 5, 2021. REUTERS/Mike Segar/File Photo)'s drug for weight loss, giving the U.S. drugmaker official entry into a lucrative market that has captured Wall Street's enthusiasm this year.
Zepbound will be available in the U.S. by the end of the year at a list price of $1,059.87, according to Lilly. That compares with a list of $1,349 per-package for Novo Nordisk - a measure of weight based on height - of at least 30, or a BMI of 27 or more if a patient also has another weight-related health issue, such as heart disease.
The Indianapolis-based drugmaker said it is launching a commercial savings card program to make Zepbound cost as little as $25 for patients whose insurance agrees to cover the drug, and $550 for those whose insurance does not. The enormous demand for new weight-loss treatments could support as many as 10 competing products with annual sales reaching up to $100 billion within a decade, mostly in the United States, industry executives and analysts said earlier this year.
Deutschland Neuesten Nachrichten, Deutschland Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA approves Eli Lilly’s Zepbound, a weight loss drug similar to Ozempic and WegovyBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
FDA approves Eli Lilly’s Zepbound, a weight loss drug similar to Ozempic and WegovyBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Weiterlesen »
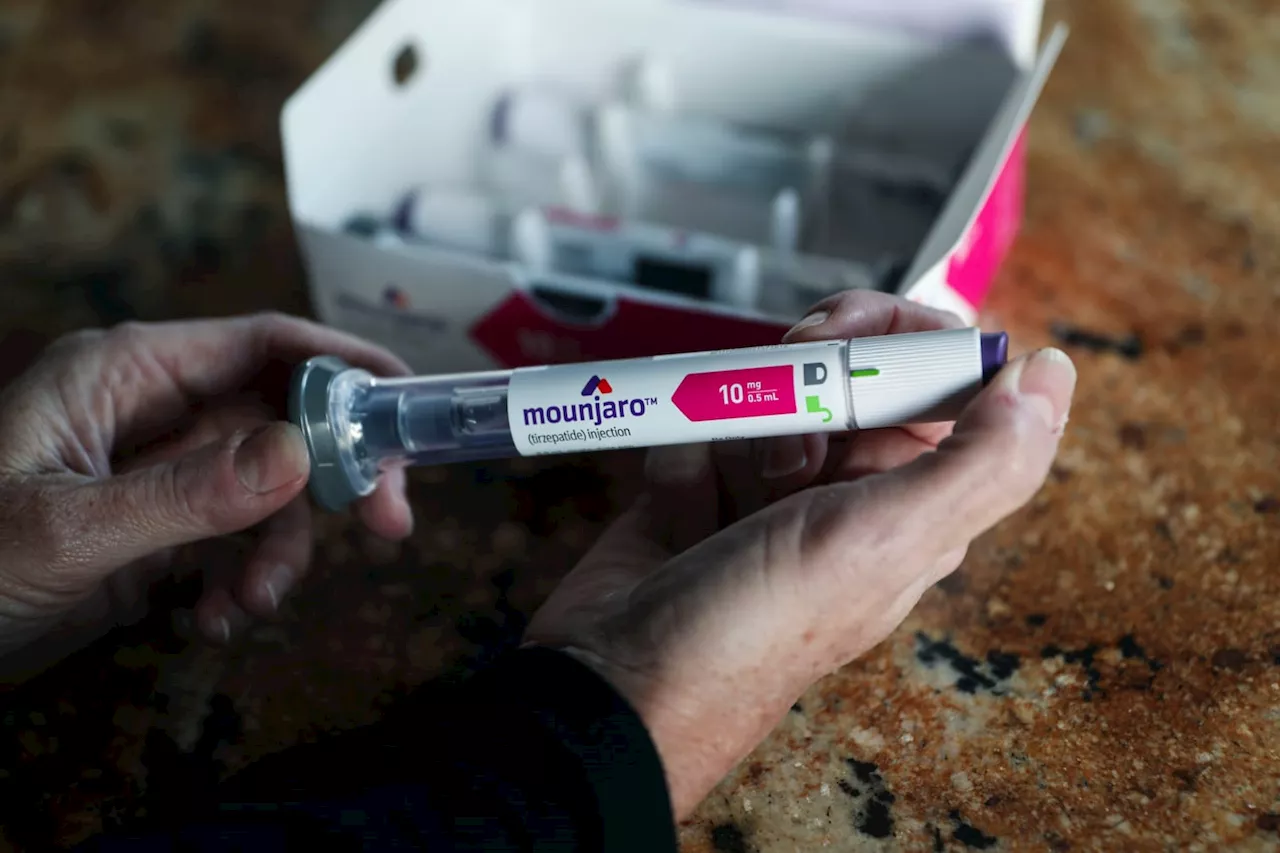 FDA approves new obesity drug from Eli Lilly named ZepboundThe decision by the Food and Drug Administration is expected to add to the demand for a new class of drugs transforming how patients battle obesity.
FDA approves new obesity drug from Eli Lilly named ZepboundThe decision by the Food and Drug Administration is expected to add to the demand for a new class of drugs transforming how patients battle obesity.
Weiterlesen »
 US FDA approves Lilly weight-loss drugThe U.S. Food and Drug Administration on Wednesday approved Eli Lilly's (LLY.N) drug for weight loss, giving the U.S. drugmaker official entry into a lucrative market that has captured Wall Street's enthusiasm this year.
US FDA approves Lilly weight-loss drugThe U.S. Food and Drug Administration on Wednesday approved Eli Lilly's (LLY.N) drug for weight loss, giving the U.S. drugmaker official entry into a lucrative market that has captured Wall Street's enthusiasm this year.
Weiterlesen »
 FDA approves a new weight loss drug, Zepbound from Eli LillyThe medication, tirzepatide, was previously approved as Mounjaro for diabetes and will now be marketed as Zepbound for weight loss.
FDA approves a new weight loss drug, Zepbound from Eli LillyThe medication, tirzepatide, was previously approved as Mounjaro for diabetes and will now be marketed as Zepbound for weight loss.
Weiterlesen »
 Eli Lilly gets FDA approval for new obesity drugZepbound helped patients lose up to 48 pounds on average in clinical trials
Eli Lilly gets FDA approval for new obesity drugZepbound helped patients lose up to 48 pounds on average in clinical trials
Weiterlesen »
 Eli Lilly's Obesity Drug Gets FDA ApprovalThe drug will be marketed as an obesity treatment under the name Zepbound.
Eli Lilly's Obesity Drug Gets FDA ApprovalThe drug will be marketed as an obesity treatment under the name Zepbound.
Weiterlesen »
